Partner with a Leading Feverfew Private Label Contract Manufacturer
Discover the benefits of working with a trusted Feverfew private label contract manufacturer. Custom solutions designed to grow your brand today!
 Get a Free Quote Today!
Get a Free Quote Today!Partnering with a feverfew private label contract manufacturer is the smartest move a health brand can make when launching a high-quality supplement. It sidesteps the monumental overhead of building your own production facility from the ground up.
This model lets you tap into our existing expertise, certified facilities, and established supply chains. We act as your R&D partner from concept through launch, turning your product vision into a market-ready reality faster and more efficiently than you could alone.
Why Smart Brands Outsource Feverfew Manufacturing
Launching a feverfew supplement brings every e-commerce founder and product manager to a crossroads: do we build our own manufacturing capabilities, or do we partner with an expert?
The idea of total in-house control sounds great on paper. But the practical realities of insane capital investment, navigating a labyrinth of regulations, and finding specialized talent make it a massive undertaking.
This is exactly why forward-thinking brands choose to outsource. Working with a specialized feverfew private label contract manufacturer isn't just about handing off production. It's a strategic partnership to accelerate growth, minimize risk, and plug into a deep well of industry-specific knowledge from day one.
Private Label vs In-House Feverfew Production
Deciding between outsourcing to a private label partner and building an in-house manufacturing arm is a critical strategic choice. For most brands, especially those focused on growth and market agility, the benefits of a partnership are overwhelming. The table below breaks down the key differences to help illustrate why outsourcing is often the smarter path.
| Factor | Private Label Partner | In-House Manufacturing |
|---|---|---|
| Initial Cost | Low; no capital expenditure for facilities or equipment. | Extremely high; multi-million dollar investment required. |
| Speed to Market | Fast; leverages existing infrastructure and processes. | Slow; 12-18+ months for facility setup and validation. |
| Regulatory Burden | Minimal; partner handles cGMP compliance and FDA regulations. | Full responsibility; requires a dedicated compliance team. |
| Expertise | Instant access to formulation and manufacturing specialists. | Must hire and train a specialized, expensive team. |
| Speed & Reliability | Rapid turnaround, transparent timelines, on-time delivery. | Must build and manage a new, unproven supply chain. |
| Focus | Brand can focus 100% on marketing, sales, and growth. | Resources are split between brand building and operations. |
Ultimately, a private label partnership allows you to concentrate your resources on building your brand and connecting with customers, leaving the complex, capital-intensive manufacturing to the experts.
Sidestep Massive Capital Investments
The cost of setting up a compliant manufacturing facility is staggering. We're not just talking about four walls and a roof; we're talking about highly specialized equipment for botanical extraction, precision filling, and rigorous quality control testing.
Just think about the essentials for a liquid supplement line:
- Extraction Vessels: You need these to process raw feverfew and isolate its active compounds, like parthenolide.
- Filtration Systems: Absolutely essential for ensuring the purity and clarity of the final liquid extract.
- Automated Bottling Lines: Critical for consistent dosing and packaging if you plan on producing at any real scale.
- Environmental Controls: These aren't just fancy air conditioners. They are sophisticated HVAC systems that maintain strict temperature and humidity levels to meet cGMP standards.
By partnering with a manufacturer, you bypass these multi-million dollar investments entirely. That capital is now freed up to focus on what you actually do best: building your brand, marketing your product, and winning over customers.
Accelerate Your Speed to Market
In the crowded supplement space, timing is everything. Trying to develop a product in-house means signing up for a long, complicated timeline. You’re looking at sourcing and vetting raw material suppliers, endless rounds of R&D, and the headache of navigating FDA registration.
A great contract manufacturer has already done all of that heavy lifting. We have solid relationships with trusted botanical suppliers, pre-validated formulas, and a deep, practical understanding of the production process. For brands that want to move quickly, this partnership is a game-changer.
The real advantage here is turning a long, resource-draining process into a streamlined, predictable launch. Instead of spending 12-18 months just building the infrastructure, you can have a finished, compliant product in a fraction of that time, with rapid, reliable turnaround.
This speed is crucial. The global feverfew extract market was valued at around USD 350 million in 2023 and is on track to nearly double by 2032. That growth is fueled by real consumer demand for natural migraine and inflammation support. You don't want to miss that wave.
Leverage Instant Regulatory Expertise
Navigating the web of cGMP (Current Good Manufacturing Practices), FDA regulations, and labeling requirements is a full-time job. One misstep can lead to expensive recalls, legal trouble, and a damaged brand reputation that's nearly impossible to repair.
An experienced manufacturer lives and breathes these guidelines every single day. We become your compliance partner, ensuring every part of your product—from the raw material's Certificate of Analysis to the claims on your final label—is meticulously managed and documented. This built-in quality and compliance provides incredible peace of mind and protects your business. To get a better handle on the entire process, check out our comprehensive guide for starting your own private label liquid supplement brand. This kind of partnership lets you focus on growth, confident that the regulatory minefield is being handled by seasoned pros.
What Actually Makes a Feverfew Extract High-Quality?
Not all feverfew products are created equal. Far from it. The difference between a supplement that delivers real results and one that just takes up shelf space starts long before anything hits a bottle. It’s all about the science, the sourcing, and the manufacturing process. If you want to build a brand that customers trust, you need to get these fundamentals right.
The whole process kicks off with the raw herb. A top-tier feverfew private label contract manufacturer doesn't just place a bulk order and hope for the best. We meticulously vet our supply. This means knowing exactly where the plant was grown, because soil and climate dramatically change its chemical makeup.
Even more critical is the testing for parthenolide. This is the main active compound—a type of molecule called a sesquiterpene lactone—that gives feverfew its powerhouse benefits. If a manufacturer can't guarantee a specific, minimum level of parthenolide, you're essentially flying blind.
Here’s a look at the plant itself, Tanacetum parthenium, where these vital compounds originate.
You're looking at the delicate flowering tops. This is where the highest concentration of parthenolide is found, and it's what any quality extract should be derived from.
The Art and Science of Extraction
Once you have premium raw feverfew, the next hurdle is getting the good stuff out. This is where a manufacturer’s technical skill really comes into play. The extraction method directly impacts the final product’s potency and bioavailability. Think of it like coffee—a slow cold brew gives you a completely different flavor and chemical profile than a fast, high-heat espresso shot.
In modern supplement manufacturing, a few advanced methods stand out:
- Cold Percolation: This is a gentle, low-temperature process. A solvent slowly moves through the raw feverfew, carefully drawing out delicate compounds like parthenolide without damaging them with heat.
- Ultrasonic-Assisted Extraction (UAE): This method uses high-frequency sound waves to literally shake the active compounds loose from the plant's cell walls. It’s highly efficient, boosting extraction yields without resorting to high temperatures.
- Supercritical CO2 Extraction: This is the high-tech option. It uses carbon dioxide under intense pressure, turning it into a "supercritical fluid" that acts as an incredibly pure solvent. It's exceptionally clean, leaving no residue.
There's no single "best" method; it depends on the end goal. An experienced manufacturing partner knows which tool to use for the job and can explain exactly why we chose it.
Keeping It Potent All the Way to the Shelf
Getting the active compounds out is only half the job. You have to protect them through the entire production process—blending, filling, and bottling. This is where cutting-edge technology separates the great manufacturers from the merely good ones.
One of the biggest differentiators you'll find is the use of cold-fill technology. This means the final liquid supplement is bottled at controlled, low temperatures. It’s a game-changer because it prevents the degradation of heat-sensitive compounds, ensuring the product your customer receives is just as potent as the day it was formulated.
This focus on innovative processing is what drives success. In fact, industry analyses show that improvements in botanical extraction have boosted feverfew's potency and purity, making it a more commercially viable and trusted supplement. As research uncovers new uses for feverfew, the market is only expected to grow. You can dive into the full market analysis from Market Research Intellect to get a better handle on the industry trends.
When you're vetting a potential partner, ask them to walk you through their process. A manufacturer who can clearly explain how every step is designed to protect the final product's potency is the one you want. They understand the science behind creating a supplement that actually works, giving you a product that builds trust and keeps customers coming back.
Understanding Compliance and Essential Certifications
Navigating the regulatory landscape can feel like a minefield, but it's the absolute bedrock of any trustworthy brand. When you're vetting a feverfew private label contract manufacturer, their certifications aren't just logos for a label. They are your brand's shield, protecting you from liability while signaling uncompromising quality to your customers.
Let's cut through the jargon and talk about the credentials that really matter. This isn't just about checking boxes—it's about understanding the deep commitment to excellence these certifications represent.

The Unbreakable Foundation: cGMP and FDA Registration
First things first: an FDA-registered facility is non-negotiable. If a potential partner isn't registered, that conversation is over before it even begins. Registration simply means the FDA knows they exist and can show up for an inspection.
But the true gold standard for quality is cGMP (Current Good Manufacturing Practices) certification. This isn't a plaque on the wall; it's a rigorous, ongoing commitment to processes that ensure your product is consistently safe, pure, and potent.
cGMP isn't a one-time award; it's a living system. It dictates every single step, from how raw feverfew is logged upon arrival to the precise calibration schedule of the bottling equipment and the air quality standards within the cleanrooms.
A manufacturer truly dedicated to cGMP can show you detailed documentation for every single batch, a concept known as traceability. This means they can track every ingredient from its original supplier all the way to the final sealed bottle on the pallet. This level of detail is your best defense against quality issues. For a deeper dive, our guide on what a cGMP certificate truly means for your brand is an invaluable resource.
Third-Party Testing: The Gold Standard for Verification
A good manufacturer has an in-house quality control lab. An elite partner takes it a step further with independent, third-party testing. For building real consumer trust and verifying the integrity of your feverfew supplement, this is absolutely essential.
This external validation serves two critical purposes:
- Potency Verification: It confirms that the level of active compounds, like parthenolide, meets the specifications printed on your label. A third-party Certificate of Analysis (CoA) is your proof that customers are getting exactly what they paid for.
- Purity Screening: It screens for contaminants that have no place in a health product. This includes heavy metals (like lead and mercury), microbes (like E. coli and salmonella), and pesticides.
Here's a pro tip: ask a potential manufacturer for recent third-party lab reports. Their willingness—and speed—in providing this documentation will tell you everything you need to know about their transparency and confidence.
Beyond the Basics: Certifications That Build Brands
While cGMP and third-party testing are the foundation, certain additional certifications can be powerful market differentiators. They speak directly to specific consumer values and can dramatically broaden your product's appeal, turning a quality supplement into a preferred choice for discerning buyers.
Think about how these certifications align with your target audience:
- UL Certified: This tells you a facility has been audited by Underwriters Laboratories, a globally respected safety science company. It’s a powerful signal of operational safety and quality management that goes beyond standard cGMP.
- USDA Organic: If your brand story is built on natural, clean ingredients, this is a must-have. It guarantees the feverfew was grown and processed without prohibited pesticides or synthetic fertilizers—a major purchasing driver for a huge segment of health-conscious consumers.
- Kosher or Halal: These certifications open your product to large, loyal consumer groups with specific dietary requirements. They involve strict audits of ingredients and production, adding another layer of quality assurance.
Choosing a feverfew private label contract manufacturer who already holds these credentials shows they're thinking ahead. They understand that today’s consumers demand not just an effective product, but also transparency and alignment with their personal values.
Your Product Development Roadmap
This is where your vision becomes a tangible, market-ready supplement. A true feverfew private label contract manufacturer isn’t just an order-taker; we become your dedicated R&D partner, guiding you from that initial idea all the way to a final, compliant, and customer-ready formula.
The journey kicks off with your unique concept. From there, we dive into the exciting work of formulation, turning your brand's goals into a precise ingredient list.

Crafting a Unique and Effective Formula
Your feverfew supplement doesn't have to be a generic product. The real opportunity is in customization—creating a proprietary blend that speaks directly to your specific audience. This is where a partnership mindset truly shines.
We'll start with foundational choices, like the base liquid. Do you want a classic alcohol tincture, known for its long shelf life? Or would a glycerin base be a better fit, offering a sweeter, alcohol-free option? Each choice has its own benefits, and we help you weigh them against your brand’s ethos and customer profile.
Next up, we explore flavor. While feverfew has its own characteristic taste, a custom flavor profile—maybe with natural mint or citrus notes—can drastically improve the customer experience and drive repeat purchases.
The most powerful differentiator is creating a synergistic blend. Think about incorporating complementary herbs like ginger, which is also studied for its role in headache support, or skullcap for its calming properties. This can elevate your formula from a single-ingredient product to a targeted, high-value solution.
This collaborative process ensures the final product isn't just effective but is also uniquely yours. As you map out the launch, integrating a well-planned product seeding strategy can be a game-changer for building early market buzz.
From Packaging Selection to Compliant Design
Once the liquid formula is perfected, we shift focus to its presentation and packaging. This step is just as critical as the formulation, balancing your brand's identity with strict regulatory compliance.
Your packaging says a lot about your brand. Will you go with a classic amber glass bottle to protect the extract from UV light? Or perhaps a modern, sleek bottle with a unique dropper cap? We’ll walk you through the options, helping you select components that are both functional and aligned with your brand's aesthetic.
Key packaging considerations include:
- Bottle Material & Color: Amber glass is the industry standard for light protection, but other options can create a more distinct feel.
- Cap & Dropper Style: A calibrated dropper with clear measurement markings adds value and ensures accurate dosing.
- Seal Integrity: Options like induction heat seals or tamper-evident sleeves provide security and build crucial consumer confidence.
At the same time, we'll get to work on the label design. This is where brand creativity meets regulatory precision. Your label has to be compelling and informative while adhering to all FDA guidelines. Our team acts as a compliance backstop, reviewing your design to make sure every required element—like the supplement facts panel and ingredient list—is accurate and correctly formatted.
Scaling Production from Pilot to Full Launch
Flexibility in production scale is a hallmark of a great manufacturing partner. Not every product launch needs to start with a massive, 100,000-unit run. We get it. You need to test the market, gather feedback, and manage your cash flow effectively.
That's why we offer a tiered approach to production, from pilot runs to full scale:
- Pilot Runs: We can start with a small batch, perfect for testing your new formula, creating marketing samples, or facilitating a soft launch to a select group of customers.
- Full-Scale Production: Once your product gains traction, we can seamlessly scale up manufacturing to meet growing demand without ever compromising on quality or consistency.
This ability to scale is crucial for any brand navigating the competitive herbal supplements market, which is part of a global industry projected to hit $65.7 billion by 2025. This flexible roadmap is designed to support you at every stage of growth—from that very first bottle to your hundred-thousandth.
How To Vet A Potential Manufacturing Partner
Picking the right partner is hands down the most critical decision you'll make for your brand. A great feverfew private label contract manufacturer becomes a true extension of your team, while the wrong one can cause endless headaches.
This process goes way beyond comparing price sheets. It's about digging in to find a partner whose operational excellence, transparency, and values truly click with yours.
The goal here is to get past the slick sales pitch. You need to ask sharp, specific questions that peel back the curtain on their actual processes. For example, asking "Can you provide a Certificate of Analysis for your feverfew raw material?" is fine. But a much better question is, "What are your quarantine and release procedures for new botanical lots?" This simple shift reveals their whole mindset around internal quality control.
Going Beyond The Facility Tour
When you're checking out a potential manufacturer, zero in on three core areas: their quality systems, their operational flexibility, and their partnership approach. A shiny facility means nothing if they have terrible communication or can't trace an ingredient back to its source.
A truly top-tier partner will not only welcome your toughest questions but will also have all the documentation ready to back up their claims.
Start by getting into the weeds on their supply chain integrity. Ask them to walk you through their supplier qualification program. How do they actually vet the farms that grow their feverfew? Do they perform on-site audits themselves? This line of questioning shows them you’re serious about quality right from the very beginning.
To really cover all your bases and minimize risk, using a comprehensive legal due diligence checklist is a non-negotiable step in the vetting process. It makes sure you've ticked all the legal and operational boxes before you even think about signing a contract.
Key Questions To Uncover The Truth
You need to arm yourself with questions that demand detailed answers, not just a lazy "yes" or "no." This framework helps you compare potential partners on the stuff that actually matters for a successful, long-term relationship.
Here are a few essential areas to probe:
- Raw Material & Traceability: "Can you provide a full traceability report for a recent batch of feverfew extract, from the raw herb's arrival to the finished product shipment?"
- Quality Control Protocols: "Describe your out-of-spec (OOS) investigation process. If a batch fails an in-process check, what are the exact steps you take?"
- Production & Capacity: "What are your typical lead times from purchase order to delivery? Can you share data on your on-time delivery rate for the past year?"
- Communication & Support: "Who will be my dedicated point of contact, and what's your standard response time for inquiries? How do you communicate production updates?"
This image breaks down what a streamlined quality control process should look like, showing how a first-rate partner ensures product integrity every step of the way.
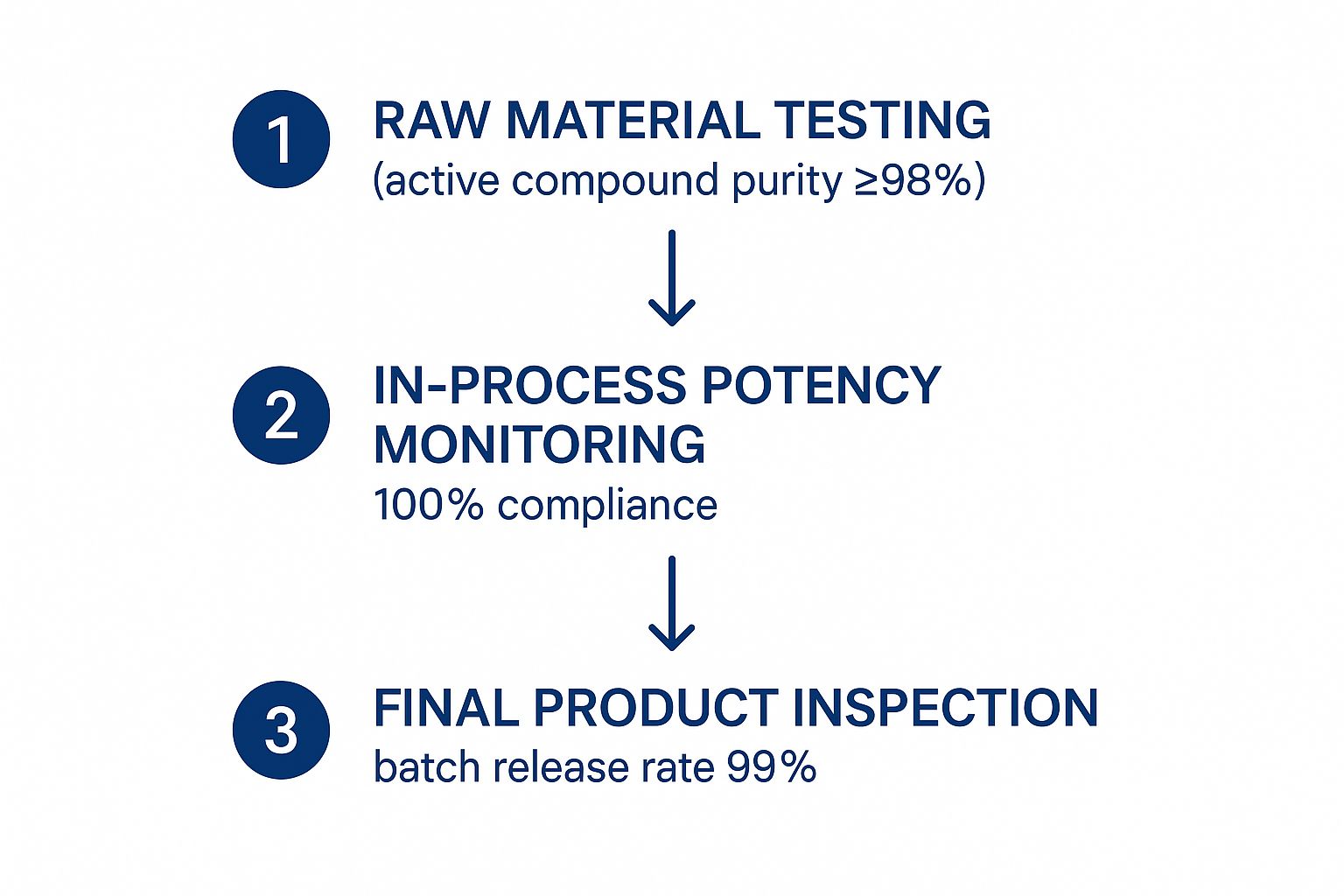
A visual like this underscores a deep commitment to excellence. When you see high purity standards for raw materials and a near-perfect batch release rate, you know they have their process control locked down.
To help you organize your questions during your vetting calls, we've put together a simple checklist.
Manufacturer Vetting Checklist
Use these essential questions to ask a potential private label partner to ensure they meet your quality, compliance, and partnership standards.
| Category | Key Question | Why It Matters |
|---|---|---|
| Sourcing & Supply Chain | Can you walk me through your supplier qualification program for feverfew? | Reveals their commitment to raw material quality from the very start. |
| Quality Control | What's your process for handling an out-of-spec (OOS) result? | Shows their problem-solving ability and adherence to cGMP protocols. |
| Compliance & Certifications | Are you FDA-registered and third-party cGMP certified? Can you provide copies? | Non-negotiable basics for any reputable manufacturer in the U.S. |
| Operational Capacity | What are your average lead times and on-time delivery rates? | Tells you if they can reliably meet your inventory needs and deadlines. |
| Partnership & Support | Who will be my dedicated point of contact? | A single, dedicated contact prevents miscommunication and streamlines everything. |
This checklist isn't exhaustive, but it's a powerful starting point to help you differentiate between a mere supplier and a true strategic partner.
Evaluating The Partnership Mindset
At the end of the day, you're not just looking for a supplier; you need a collaborator. A real partner is invested in your success. They should be proactive, offering suggestions for formula improvements or flagging potential supply chain hiccups before they become real problems.
The best manufacturing relationships are built on a shared commitment to quality and growth. Does the manufacturer seem genuinely interested in your brand's vision, or do they see you as just another order to fill? Their enthusiasm and willingness to act as an extension of your R&D team is often the clearest indicator of a strong future partnership.
This vetting process takes time and diligence, but the payoff is huge. For a wider view of what makes these relationships work, our overview of nutraceutical contract manufacturing offers some great additional context. Finding that perfect fit gives you the confidence to focus on what you do best—building your brand—knowing the complex manufacturing is in expert hands.
Common Questions About Feverfew Manufacturing
Jumping into the world of private label supplements always brings up questions. As a long-standing feverfew private label contract manufacturer, we believe being upfront is the only way to build a great partnership. Getting your practical concerns answered from the start helps you plan your launch with confidence.

Here, we tackle the most common questions we hear from ambitious entrepreneurs. Our goal is to pull back the curtain on the process so you can move forward with total clarity.
What Are Typical Minimum Order Quantities?
Let's talk about MOQs, or minimum order quantities. This is a huge consideration, especially when you're just starting out. The honest answer is: it depends. MOQs are dictated by the complexity of your formula and the uniqueness of your packaging.
A simple, single-herb feverfew tincture using standard bottles might have an accessible MOQ, often starting around 500 to 1,000 units. This is a great way for new brands to test the market without sinking a massive investment into inventory.
However, if you're creating a complex blend with custom flavors or unique packaging that we have to special order, the MOQ will naturally be higher. A flexible partner will work with you on run sizes to find a sweet spot that balances your budget with the realities of production.
What Is a Realistic Production Timeline?
So, your formula is locked in and your label design is approved. What’s a realistic timeframe to get your finished product in hand? For us, speed and reliability are everything, and a transparent timeline is a non-negotiable part of our partnership.
Generally, a production schedule looks something like this:
- Material Sourcing & Testing (1-2 weeks): We procure all raw ingredients, including the feverfew extract, and immediately run identity and purity tests.
- Manufacturing & Bottling (1-2 weeks): Your product is batched, blended, and bottled right here in our cGMP-certified, FDA-registered facility.
- Third-Party Lab Testing (1 week): The finished product is sent to an independent lab to verify its potency and purity.
- Labeling & Final QC (1 week): Your bottles are labeled, packed, and put through one last quality assurance check before they head out the door.
From the moment you give final approval to the day we ship, a typical production run takes about 4-6 weeks. This rapid turnaround ensures we never cut corners on quality control while still moving efficiently to get your product to market.
Who Is Responsible for Label Compliance?
This is a critical question, and the answer is that it's a partnership. At the end of the day, you, the brand owner, are legally responsible for every single claim made on your product's label. The FDA holds the brand accountable for what the product is, what it contains, and what you claim it can do.
However, a true manufacturing partner doesn't just leave you to figure it out alone. We act as your expert compliance guide. We'll review your label design from top to bottom to make sure it meets all FDA requirements for dietary supplements. This means checking the supplement facts panel format, ingredient list, and other required statements.
We're here to help you sidestep common mistakes that could put your brand at risk, ensuring your packaging is 100% ready for retail, whether you're selling on Shopify or through Amazon FBA.
Ready to turn your vision for a premium feverfew supplement into a market-ready reality? At Triton Nutra Group, we become your dedicated R&D and manufacturing arm from concept through launch. Let's build something exceptional together. Request your free consultation and quote today!