Partnering with a GMP Certified Facility for Your Liquid Supplements
Discover why a GMP certified facility is critical for your supplement brand's quality, safety, and success. Learn how to choose the right manufacturing partner.
 Get a Free Quote Today!
Get a Free Quote Today!Choosing a manufacturing partner is the single most important decision you'll make for your supplement brand. A GMP certified facility isn't just a vendor; it's the foundation of your entire business. This partnership is the unseen guarantee that every bottle you sell is safe, consistent, and effective.
Why a GMP Certified Facility Is Your Brand's Foundation
In the health and wellness space, customer trust is everything. Good Manufacturing Practice (GMP) certification is the globally recognized gold standard that proves your partner is serious about quality control.
This isn't just a suggestion; it's a formal system of controls designed to protect against contamination, ingredient mix-ups, and any error that could put your customers—and your company's reputation—at risk.
The roots of GMP go back to 1968 when the World Health Organization (WHO) adopted the first draft guidelines. Today, well over 100 countries have integrated these principles into their national laws, highlighting their global importance.
More Than Just a Certificate on the Wall
For you, the brand founder, partnering with a GMP certified facility brings tangible benefits. This certification is a clear signal that your manufacturer follows strict, documented protocols for:
- Facility Cleanliness: Ensuring the entire production environment is sanitized and meticulously controlled to prevent contamination.
- Equipment Maintenance: Using properly calibrated and maintained equipment to ensure every batch is identical to the last.
- Raw Material Sourcing: Verifying the identity, purity, and quality of every single ingredient before it enters your formula.
- Employee Training: Requiring rigorous, ongoing training so every person on the line understands and follows standard operating procedures without deviation.
Think of it this way: GMP compliance is the difference between a professional kitchen with a health department "A" grade and a home cook just winging it. One delivers predictable, safe results every single time; the other is a complete gamble.
Ultimately, choosing a GMP certified facility is a foundational business move. It slashes your risk, builds unshakable consumer confidence, and establishes a standard of quality that sets your brand apart. For any serious player in the supplement industry, it's non-negotiable.
To learn more about the specifics, see what a GMP certificate means for your supplement brand.
Decoding cGMP Compliance for Liquid Supplements

You’ve seen "GMP" on a manufacturer's website. But the most important letter to look for is 'c'.
That 'c' stands for current, and it makes all the difference. The distinction between GMP and cGMP is simple but profound. It signals that your manufacturing partner isn’t just following a dusty rulebook. A cGMP certified facility is actively committed to the most up-to-date quality standards and technologies the FDA enforces.
It’s an ongoing promise of excellence, not a one-time checkbox.
For liquid supplements, especially herbal formulas, this forward-thinking approach is critical. The industry is constantly innovating with new botanical extraction methods and stability testing. Being 'current' means your products are made with the best practices available today.
From Rules on a Page to Quality in a Bottle
Regulatory language can be dense, but cGMP principles aren't just red tape; they're a robust quality system protecting your brand and your customers.
Here's what that looks like in practice:
- Validated Processes: This is proof that the production steps work. Every single time. It means your elderberry syrup has the same potency and flavor profile in the first bottle as it does in the ten-thousandth. No guesswork allowed.
- Raw Material Quarantine & Testing: Before a single botanical ingredient gets near the production line, it’s held in quarantine. It remains there until it passes rigorous third-party tests for identity, purity, and potency. This stops a bad batch of herbs from ever compromising your formula.
- Cross-Contamination Prevention: A true cGMP facility is meticulously designed with strict controls for airflow, personnel movement, and sanitation schedules. This is critical for preventing allergens from one product ending up in another, ensuring label accuracy and consumer safety.
Simply put, cGMP is the operational blueprint that transforms a good product idea into a consistently safe and effective supplement. It’s the behind-the-scenes system that builds long-term consumer trust.
The table below breaks down these core requirements and shows you exactly how they shield your brand from risk.
Core cGMP Requirements and Brand Benefits
| cGMP Requirement | What It Means in Practice | How It Protects Your Brand |
|---|---|---|
| Personnel & Training | Staff are fully trained on their specific roles, hygiene protocols, and quality control procedures. | Reduces the risk of human error that could lead to contamination or inconsistent batches. |
| Facility & Equipment | The physical plant is clean, well-maintained, and designed to prevent mix-ups. Equipment is calibrated and sanitized on a strict schedule. | Ensures a stable, controlled environment, preventing your product from being exposed to contaminants or allergens. |
| Raw Material Control | All incoming ingredients are quarantined, tested for identity and purity, and formally approved before use. | Guarantees that what's on your label is actually in the bottle, starting with pure, high-quality ingredients. |
| Production & Process Controls | Every step of the manufacturing process is documented and validated to ensure consistent outcomes. | Delivers product uniformity from batch to batch, so your customer gets the same experience every time. |
| Documentation & Records | Meticulous records are kept for every batch, from raw materials to final distribution (often called a Batch Production Record). | Creates full traceability. In the event of a quality issue, you can quickly identify the source and scope of the problem. |
| Testing & Quality Control | Finished products are tested to confirm they meet all specifications for potency, purity, and composition before being released. | Verifies that the final product is safe, effective, and matches the claims you make on your label. |
As you can see, each regulation is a layer of protection. This system is what separates professional, reliable manufacturing from the rest.
The Rigorous Path to Certification
Earning a cGMP certification isn't a weekend project; it’s an exhaustive, ongoing commitment. The process involves meticulous documentation reviews, tough on-site audits by organizations like UL, and thorough inspections of the FDA-registered facility.
A GMP certified facility must prove it maintains spotless premises, employs qualified people, uses validated processes, and runs reliable product testing. Failure to keep up can lead to recalls or legal trouble, which is why any serious manufacturer makes compliance their top priority. You can get more insights on the latest current GMP guidelines and their importance on pharmuni.com.
Ultimately, these strict rules give you the confidence to stand behind your products. When you partner with a manufacturer that lives and breathes cGMP, you’re not just buying a production run—you're investing in a promise of quality that becomes your brand's foundation.
The Real Business Benefits of a GMP Partnership

Choosing a manufacturer is more than a line item on your budget. It's a strategic decision that directly impacts your brand's growth. When you work with a true GMP certified facility, you’re not just checking a compliance box—you’re gaining a competitive edge.
The benefits boil down to three things every e-commerce founder cares about: Quality, Speed, and Partnership. Imagine launching a new product with total confidence, knowing it came from an FDA-registered, UL certified process and was verified by third-party testing. That peace of mind drastically cuts the risk of a brand-damaging—and expensive—product recall.
It also means you get predictable production timelines and fewer batch failures. This makes managing inventory and cash flow much easier. A reliable partner removes the guesswork, freeing you to focus on marketing and sales instead of fighting supply chain fires.
Driving Quality and Reducing Risk
The most immediate payoff of a GMP partnership is risk mitigation. Every step is designed to lock in safety and consistency, which translates directly into financial protection for your brand. This isn't just about dodging bullets; it's about building a rock-solid reputation for excellence from day one.
This is accomplished through key practices:
- Meticulous Record-Keeping: A full batch production record (BPR) is created for every run. This gives you complete traceability, from raw materials to finished product.
- Third-Party Testing: Independent labs verify the potency and purity of your final product. You get an unbiased certificate of analysis, which is gold for building consumer trust.
- Reduced Batch Failures: With validated processes and strict quality controls, fewer production runs go to waste. That saves you significant time and money.
Accelerating Speed to Market
In the fast-paced world of e-commerce, speed and reliability are everything. A top-tier GMP certified facility operates with the efficiency and transparent timelines you need to scale your brand. This operational excellence means you can jump on market opportunities without being slowed by production delays.
A true partner helps you move faster by offering flexible run sizes—from pilot to full-scale production—and a streamlined process from formulation to fulfillment. You can launch, test, and scale more effectively with a reliable partner who has a proven on-time delivery record.
A partnership with a GMP certified facility is an investment in your brand's operational backbone. It provides the stability and quality assurance needed to grow confidently, knowing your supply chain is secure and your products are unimpeachable.
Your manufacturer should feel like an extension of your own team—your R&D partner from concept through launch. They can provide invaluable guidance on complex formulations, help you navigate regulatory hurdles, and offer the flexibility you need as you grow. This collaborative approach saves immense time and resources, letting you bring your vision to life faster.
How To Evaluate Your Manufacturing Partner
A GMP certificate is the baseline—the ticket to the game, not the win. Any serious manufacturer will have one. Your job as a brand founder is to look beyond that paper and find a true partner who understands the nuances of your product, especially if it’s a liquid herbal supplement.
The key is asking the right questions. This process separates a basic supplier from a collaborative partner who can help you innovate. It’s not just about vetting their compliance; it’s about confirming they have the expertise, customization flexibility, and forward-thinking approach your brand needs to thrive. The right partner becomes an extension of your team.
Think of this evaluation as your chance to gauge their depth of knowledge and true commitment to quality.
Questions That Go Beyond The Certificate
When you start conversations with a potential GMP certified facility, your goal is to understand their real-world capabilities. Don't be shy about digging into their processes and experience.
Here are the critical areas to explore:
- Liquid Herbal Expertise: Do they have specific, demonstrable experience with botanical extracts and liquid formulations? Ask for examples of similar products they've made. Vague answers are a red flag.
- Raw Material Sourcing: How do they vet their botanical suppliers? Request details on their qualification process and how they verify the identity and purity of raw herbs before they enter your product.
- Batch Size Flexibility: Can they support your brand's entire journey? You need to know their capabilities for small pilot runs for market testing, as well as their capacity for full-scale production as you grow.
And don't forget the basics of facility management. Their commitment to maintaining a sterile environment is paramount. This includes understanding their protocols for things like specialized manufacturing facility cleaning services, which are fundamental to preventing contamination and ensuring your product's integrity.
Identifying Innovative Differentiators
A good partner meets the standards. A great partner pushes them. Look for signs of innovation that show a manufacturer is invested in producing the absolute best product for you.
This simple decision tree illustrates the core quality control checkpoints that should exist within any top-tier GMP facility, from raw materials to the final product.
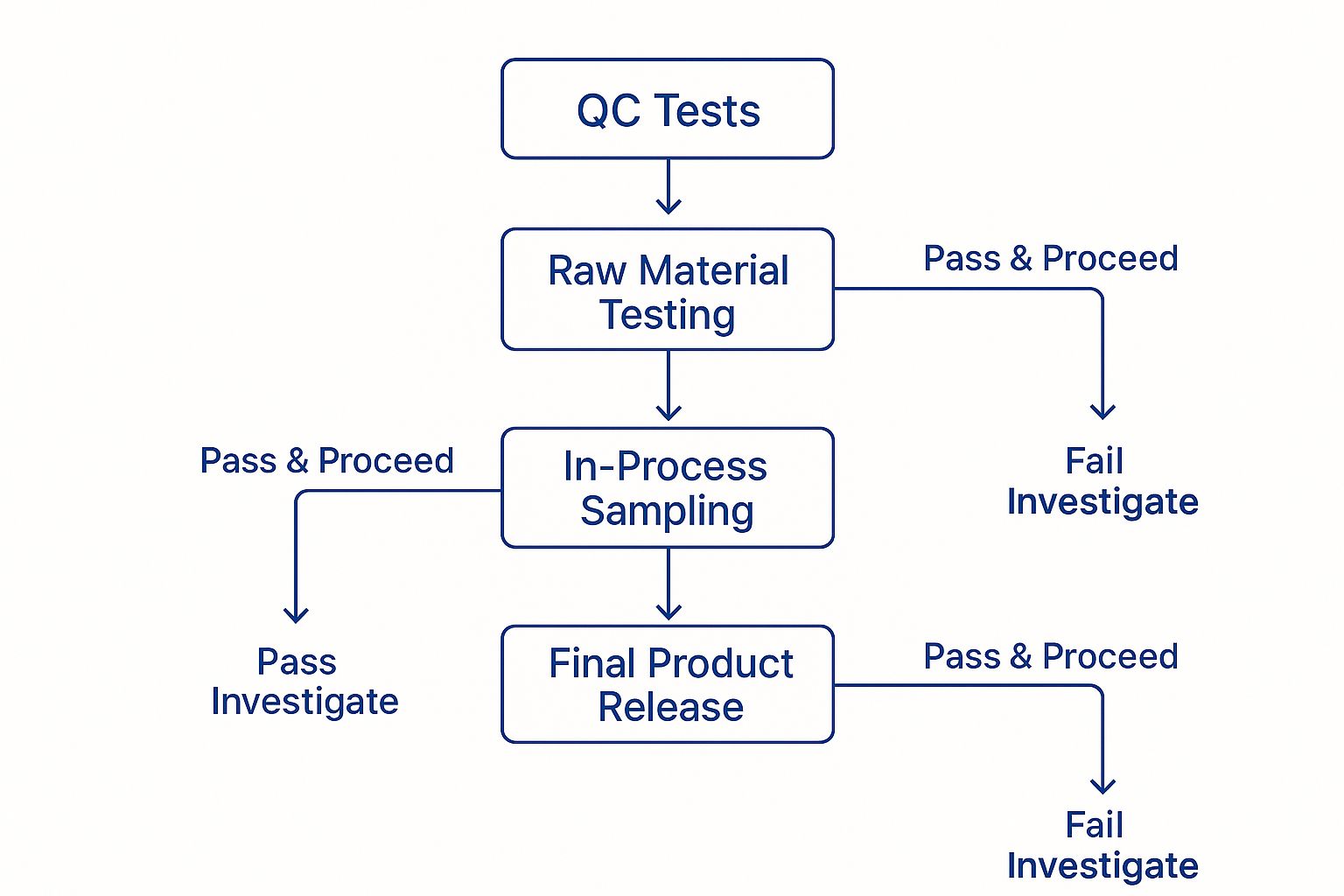
As you can see, a robust quality system relies on multiple testing gates. This ensures that any deviation is caught immediately, preventing a compromised product from ever reaching the market.
This dedication to quality often shows up in their technology. Do they use advanced, temperature-controlled botanical extraction methods to preserve delicate active compounds? Do they employ innovative cold-fill technology to protect the integrity of heat-sensitive ingredients? These aren't just buzzwords; they are clear markers of a manufacturer dedicated to superior quality.
To help you stay organized, we've put together a simple checklist. Use it to compare potential partners side-by-side.
Manufacturer Evaluation Checklist
| Evaluation Area | Key Questions to Ask | What to Look For in the Answer |
|---|---|---|
| Specialization | Do they focus on liquids, or are they a generalist? What percentage of their business is liquid herbals? | Look for a specialist. A manufacturer who primarily deals in liquids will have deeper expertise and more suitable equipment. |
| Sourcing Transparency | Can they provide full documentation for raw material origins and testing? Can you see a Certificate of Analysis (COA) for a recent batch? | You want complete transparency. They should be able to trace every ingredient back to its source with full documentation readily available. |
| Scalability | What are their minimum and maximum run sizes? Do these align with your current needs and your five-year growth plan? | A good partner can handle small test batches and scale up with you. Inflexible batch sizes can stifle growth or create inventory issues. |
| Technology & Innovation | What advanced processes (e.g., cold-fill, specific extraction methods) do they use to enhance product quality and stability? | Listen for specific technologies that protect ingredient integrity. This shows they are investing in quality, not just meeting minimum standards. |
| Partnership Mindset | In your conversations, do they sound like a vendor just taking an order, or a collaborator invested in your brand's success? | Seek a true partner. They should ask insightful questions about your brand and offer suggestions to improve the product or process. |
Choosing the right manufacturer is a decision that will ripple through every aspect of your business. For a more detailed breakdown, check out our guide on how to find a reputable supplement manufacturer.
By asking these focused questions, you can confidently select a partner who will help build your brand on a solid foundation of quality and reliability.
Your Product Journey From Concept to Launch
Let's pull back the curtain on how to bring a supplement to life with a manufacturing partner who's truly in your corner. A GMP certified facility isn't just an order taker; they function as an extension of your team, guiding your product from a spark of an idea to a launch-ready reality.
Imagine you have a concept for a new liquid ashwagandha blend aimed at stress relief. That's where it all begins. We'll follow this product through each critical stage to show how a complex process becomes manageable and reliable.
Stage 1: The Custom Formulation
It starts with your vision. You bring us the concept, and our formulation experts translate that vision into an effective formula. This is a collaborative process where we talk through ingredient profiles, nail down optimal dosages, and even explore synergistic botanicals to give your product an extra edge.
This is more than just mixing ingredients. We meticulously ensure the final formula is compliant, shelf-stable, and can be manufactured at scale. Your idea is transformed into a precise scientific blueprint.
Stage 2: Flavoring and Pilot Batches
With a solid formula, the next step is perfecting the taste. Our R&D team develops custom flavor profiles that work with the natural herbal notes, ensuring your supplement is not just potent but also enjoyable.
Once you’ve signed off on a flavor, we produce a small pilot batch. This is a crucial dress rehearsal, not a full production run. This small-scale version allows you to sample the finished product, share it with stakeholders, and make final tweaks before committing to a major inventory investment.
The pilot stage is where your idea finally becomes tangible. It’s the first time you get to hold a bottle of your product—a massive milestone that closes the gap between a formula on a spreadsheet and a real-world supplement.
Stage 3: Stability Testing and Packaging
While you're testing the pilot batch, we're running rigorous stability trials in the background. These tests are essential for determining your product's shelf life. They confirm it will maintain its potency and safety right up to the expiration date—a non-negotiable step for any reputable brand.
At the same time, we're finalizing packaging. We help ensure your labels meet all FDA requirements and that your chosen bottles, caps, and seals are a perfect match for the formula, protecting its integrity all the way to your customer's doorstep.
Stage 4: Full-Scale Production and Launch
With the formula locked, flavor perfected, and stability confirmed, it's go-time. We move into full-scale production with a rapid, transparent timeline. Because we followed a meticulous, GMP-compliant process from the start, scaling up is a smooth transition. Our systems are built to ensure that the 100,000th bottle is identical in quality to the first one from your pilot batch.
From concept to your first production run, we provide the expertise and ensure compliance every step of the way. We handle the complexities so you can stay focused on what you do best: building your brand.
To see how this hands-on approach fits into the bigger picture, check out our guide on how to choose a liquid supplement manufacturer.
Common Questions About GMP Manufacturing

As a brand founder, you’re focused on growth, marketing, and the big picture. Navigating the technical side of manufacturing can bring up a lot of questions.
Getting straight, simple answers is the only way to make smart decisions for your business. Here, we tackle the most common questions we hear from brand owners just like you.
What's the Real Difference Between GMP and cGMP?
This is one of the most frequent questions, and the answer is in the letter 'c'. While GMP stands for Good Manufacturing Practices—the basic quality standard—the 'c' in cGMP stands for current.
That single word changes everything.
Being current means a facility can't rest on old standards. It must stay on top of the latest regulations, technologies, and scientific understanding. Think of it this way: GMP is the rulebook, but cGMP means you're playing by the most recent version of the rulebook. The FDA enforces cGMP to ensure manufacturers are using modern, robust systems to prevent contamination, ingredient mix-ups, and other costly errors.
For you, this means a cGMP partner is committed to the highest contemporary standards for safety and quality.
Does GMP Certification Make My Product More Expensive?
It’s a fair question. Will partnering with a top-tier GMP certified facility drive up your costs? The short answer is that while a fully compliant facility might have a higher price tag than a non-certified one, it's an investment that saves you a fortune down the road.
Think about the catastrophic costs of a product recall, a lawsuit, or the permanent damage to your brand's reputation from a single quality failure. Those financial hits would dwarf the initial investment in doing things right.
Plus, cGMP-compliant facilities are more efficient. They have fewer rejected batches, more reliable production schedules, and less waste. This operational excellence creates cost stability and predictable timelines, making inventory management and financial planning much easier.
Choosing a cGMP-compliant partner isn't a cost center; it's an insurance policy for your brand's reputation and financial health. It shifts the focus from short-term price tags to long-term value and stability.
Can a Startup Even Work with a GMP Facility?
Can a small startup realistically team up with a high-level GMP certified facility? The answer is a resounding yes. In fact, the best manufacturers specialize in working with brands of all sizes.
The secret is finding a partner that values flexibility. Look for a facility that can handle smaller pilot batches to test the market and then scale production seamlessly as your sales take off.
A true partner will act as a collaborator, not a simple order-taker. They'll help you navigate the process, communicate their minimum order quantities (MOQs) clearly, and show a real interest in growing with you.
Ready to turn your vision for a liquid supplement into a market-ready success? The experts at Triton Nutra Group are your dedicated partners from concept to launch. Request a free quote today and let's build your brand on a foundation of quality.