What Does GMP Certified Mean for Your Supplement Brand?
Discover what does GMP certified mean for your brand. Learn how these manufacturing standards build trust, ensure quality, and protect your customers.
 Get a Free Quote Today!
Get a Free Quote Today!For a brand manager or e-commerce founder in the health and wellness space, GMP certification is your non-negotiable promise of quality. It’s the framework that ensures every single product is manufactured to the highest possible standards for safety, purity, and consistency. Think of it as the ultimate quality control system, the one that protects your customers—and your reputation—from day one. It’s your guarantee that the formula you meticulously approved is the exact formula that reaches their hands, every single time.
What GMP Certified Means for Product Quality and Trust

For any entrepreneur launching a supplement brand, truly understanding what GMP certified means is table stakes. This is much more than a seal on a package; it's a comprehensive framework ensuring your manufacturing partner operates at a level that guarantees product integrity. The "GMP" stands for Good Manufacturing Practices, a set of incredibly stringent guidelines enforced by regulatory bodies like the FDA.
You'll also see the term "cGMP," where the 'c' stands for 'current.' This distinction is vital. It means a manufacturer must use up-to-date technologies and systems, not just rely on methods that were good enough five years ago. It signals a deep commitment to continuous improvement and innovation, which is critical in a fast-evolving industry.
The Foundation of Trust and Consistency
Picture a cGMP-certified facility as a high-tech, impeccably clean lab. Every single raw ingredient is meticulously tested before it even enters the production line. This crucial first step, which we detail in our guide on supplement ingredient testing, is all about ensuring purity and potency from the start.
Inside this facility, every step of the manufacturing process follows a precise, documented recipe—what we call a Standard Operating Procedure (SOP). This covers everything from how equipment is cleaned and calibrated to how staff are trained and how individual batches are tracked. This obsession with process delivers one key benefit above all others: unwavering consistency.
A GMP certification provides assurance that the product's manufacturing meets high-quality standards. It fills a crucial regulatory gap by holding facilities accountable for accuracy, safety, and quality control.
A Global Standard for Excellence
Good Manufacturing Practice isn't just a local standard; it’s a global benchmark for quality. Its formal structure began taking shape back in 1968 when the World Health Organization (WHO) adopted its first draft text. Today, over 100 countries have integrated WHO GMP provisions into their national laws, cementing its role in international quality assurance.
For e-commerce founders, this is a powerful strategic advantage. Partnering with a cGMP-certified manufacturer like Triton Nutra Group immediately demonstrates a commitment to quality that builds trust with customers and ensures your products meet the rigorous standards of major online marketplaces. To get a feel for the bigger picture, exploring a guide to global compliance certification can show how businesses meet internationally recognized rules for quality and safety, much like GMP.
The Core Pillars of A GMP-Compliant Facility

To really grasp what GMP certified means, you have to look past the certificate and see the system in action. A compliant facility isn’t just a clean building. It’s a living ecosystem where every detail is obsessively managed to produce a perfect, repeatable result.
This entire framework rests on what industry insiders call the "5 P's"—a set of interconnected pillars that ensure nothing is left to chance. For brand owners, understanding these pillars gives you a crystal-clear checklist for vetting any potential manufacturing partner.
People and Premises
The first two pillars are the most tangible: the people doing the work and the space where they do it.
A true GMP facility invests heavily in its team. Staff are highly trained professionals who undergo continuous education on everything from hygiene and equipment operation to contamination prevention. Their expertise is your first line of defense against costly mistakes.
Equally critical is the Premises. This goes far beyond a basic janitorial schedule. We're talking about purpose-built cleanrooms with controlled airflow and pressure to stop cross-contamination. Every surface is designed for easy sanitization, and equipment is calibrated with surgical precision to guarantee consistency from one batch to the next.
Products, Processes, and Procedures
The final three pillars are all about the manufacturing workflow itself. They’re what make sure every action is intentional, documented, and verifiable.
-
Products: This covers the entire lifecycle of your supplement, from uncompromising quality control on raw materials to the final packaged goods. Every ingredient is tested for identity, purity, and potency before it gets near a mixing tank.
-
Processes: GMP requires validated, repeatable manufacturing steps. For liquid supplements, that means using advanced methods like our cold-fill technology and temperature-controlled botanical extraction, which carefully preserves delicate compounds that heat can destroy. This ensures maximum efficacy.
-
Procedures: These are the detailed Standard Operating Procedures (SOPs) for every task. Think of it as the facility’s operating system—an exhaustive rulebook that dictates everything from how a batch record is filled out to how a spill is cleaned.
The essence of GMP compliance is building quality into the system at every stage, not just testing for it at the end. It's a proactive strategy that makes consistency the inevitable outcome.
To help you visualize this, here’s a quick breakdown of how these principles apply at each stage of production.
GMP Compliance Checklist From Raw Materials to Final Product
| Compliance Area | What It Means for Your Product | Example Action |
|---|---|---|
| Raw Material Control | Ensures every ingredient is pure, potent, and exactly what it claims to be. | Quarantining and testing a shipment of Ashwagandha for identity and contaminants before releasing it for production. |
| Facility and Equipment | Prevents contamination and guarantees that machinery operates correctly every time. | Daily calibration of scales and environmental swabbing of cleanroom surfaces to test for microbes. |
| Personnel Training | Reduces the risk of human error, a leading cause of batch failure. | Documented training for technicians on proper gowning procedures and SOPs for new equipment. |
| Manufacturing Process | Guarantees every batch is made using the exact same validated steps. | Following a master manufacturing record (MMR) that details every step, from ingredient weighing to mixing times. |
| Documentation & Records | Creates a complete, traceable history of your product from start to finish. | Maintaining detailed batch production records (BPRs) that log every action, signature, and measurement for a specific lot. |
| Finished Product Testing | Verifies the final product meets all specifications for safety, purity, and potency. | Sending samples from a completed batch to a third-party lab to confirm it's free of heavy metals and microbes. |
This meticulous approach gives you full traceability. If a question ever arises about a specific batch, a cGMP-certified manufacturer can pull detailed records and trace its entire history. This level of documentation is central to understanding the full scope of dietary supplement manufacturing requirements and is non-negotiable for protecting your brand.
Why GMP Certification Protects Your E-Commerce Brand
In the hyper-competitive e-commerce world, your brand's reputation is your most valuable asset. A single bad batch or a flood of one-star reviews can unravel all your hard work overnight. This is why what GMP certified means isn't just about quality control—it’s your single most powerful tool for risk management.
By partnering with a cGMP-certified manufacturer, you dramatically slash the risk of devastating product recalls, unwanted attention from the FDA, and the kind of viral customer complaints that can tank a business. It’s a direct investment in your brand’s stability and your peace of mind.
Navigating a Landscape of Increasing Scrutiny
The digital marketplace is no longer the Wild West. Major online retailers like Amazon have dramatically tightened their compliance requirements for dietary supplements. For them, GMP certification isn't a "nice-to-have"; it's a non-negotiable key to market access. Trying to sell products from a non-certified facility on these platforms is often a complete non-starter.
These retail titans know their reputation is on the line with every product they list. To protect themselves, they are rigorously demanding proof of GMP compliance.
For an e-commerce brand, a GMP certificate is your passport to major sales channels and a powerful signal of credibility to both platform auditors and discerning customers.
Navigating this web of rules requires a solid grip on the bigger picture. This broader context of understanding retail compliance highlights why a foundational certification like GMP is so critical for any brand that wants to thrive online.
From Expense to Strategic Investment
Ambitious entrepreneurs often see the costs of partnering with a top-tier manufacturer as a major expense. That's a mistake. The right way to frame it is as a strategic investment in the long-term health and sustainable growth of your business.
Here’s the breakdown:
- Minimizes Financial Risk: The cost of a single product recall can be catastrophic. GMP compliance is your insurance policy against this exact scenario.
- Builds Lasting Customer Loyalty: Today’s consumers are savvy. They research brands and actively seek proof of quality. Showing your commitment to cGMP manufacturing builds a loyal customer base.
- Unlocks Scalability: As your brand grows, you need a partner whose processes are documented, repeatable, and ready to scale. GMP provides the exact framework that makes reliable, large-scale production possible without sacrificing quality.
Ultimately, choosing a cGMP-certified manufacturer is a proactive business decision that defends your brand, secures access to the biggest markets, and builds an unshakeable foundation of trust with your customers.
How to Verify GMP Audits and Certifications
A certificate on a wall is only as good as the authority that issued it. For any e-commerce founder, knowing how to vet a potential manufacturing partner’s GMP certification is non-negotiable. It’s how you protect your brand by ensuring their commitment to quality is proven, transparent, and actively maintained.
Asking the Right Questions
First, understand the difference between an internal audit and a legitimate third-party certification. While internal audits are a standard part of good supplement quality control, they lack the unbiased rigor of an independent inspection.
The gold standard is a certificate from a respected, accredited third-party body like UL, NSF International, or the Natural Products Association (NPA). These groups conduct exhaustive, on-site audits to verify every aspect of a manufacturer’s operations. When evaluating a partner, don’t just ask, "Are you GMP certified?" Dig deeper. A trustworthy manufacturer will be transparent and ready to show you the proof.
Here are the essential documents you need to request and review:
- A copy of their current GMP certificate: Check the issuer and, most importantly, the expiration date. These certifications require regular renewal.
- The scope of the certification: Ensure the certificate specifically covers the product type you want to make, like liquid dietary supplements.
- The most recent audit report summary: A summary can provide incredible insight into their compliance level and any notes from the auditor.
A manufacturer who is proud of their quality systems will have this information ready. Any hesitation or refusal to share these basic documents is a massive red flag.
This isn't just about ticking boxes. As the visual below shows, GMP compliance has a direct, tangible impact on your business outcomes, steering you toward sustainable growth.
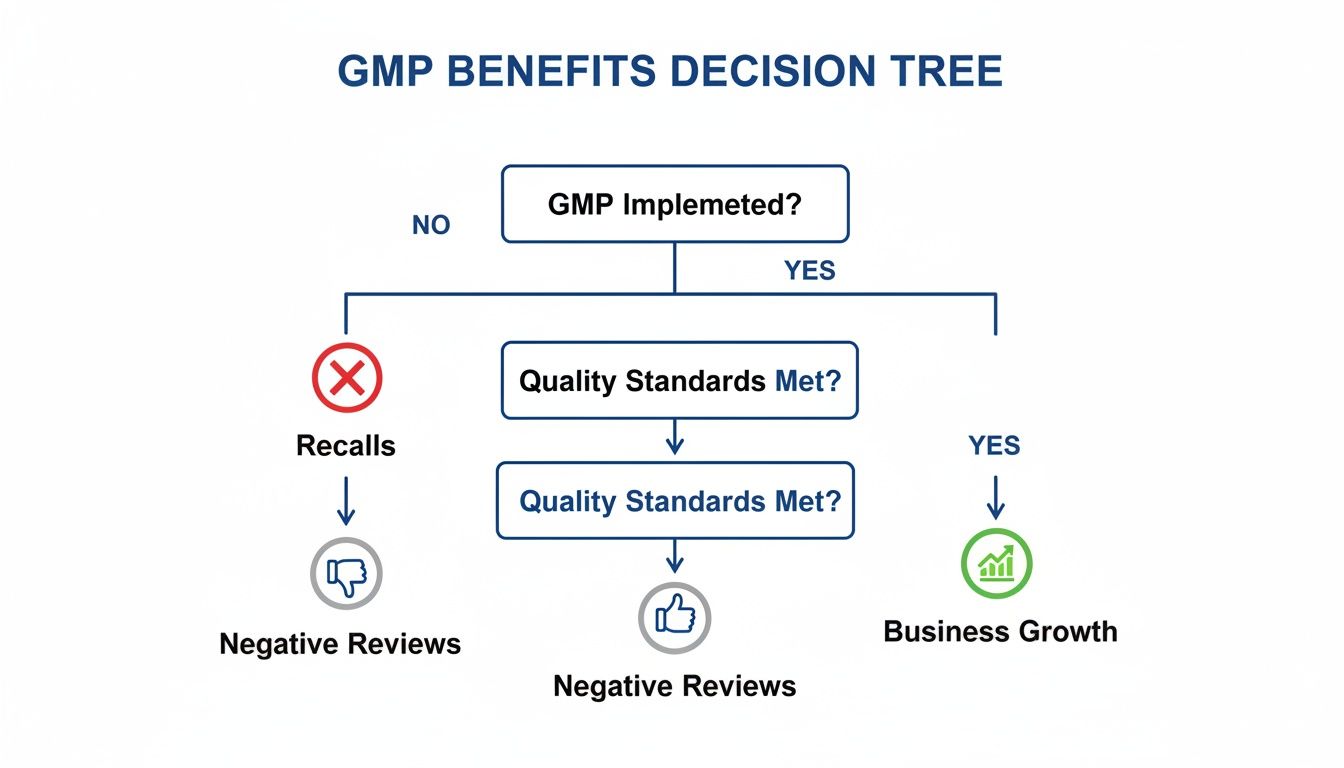
This decision tree makes it clear: strong quality standards aren't just about following rules. They're a direct line to minimizing risk and building a brand that lasts.
Verifiable Proof Is Everything
The weight of these standards is felt globally. The EMA (European Medicines Agency), for example, requires any medicine sold in the EU to meet EU GMP standards, no matter where it was manufactured. This shows just how powerful and far-reaching GMP compliance is.
Ultimately, verification is about building trust through objective proof. Your manufacturer should be your R&D and production partner, and any solid partnership must be built on a foundation of proven quality.
Common Myths About GMP Certification Debunked
When building a brand, misinformation can lead to expensive mistakes. Let's cut through the noise and tackle the most common myths entrepreneurs have about GMP certification so you can make smarter decisions.
One of the biggest misunderstandings is that "GMP certification guarantees a product's effectiveness." That’s not its job. Knowing what GMP certified means is understanding its purpose: it guarantees the process, not the clinical outcome. GMP ensures your supplement is produced safely, consistently, is free from contaminants, and contains exactly what the label says it does. It’s a promise of safety and quality, not specific health results.
Beyond the Big Corporations
Another myth is that "GMP is only for huge corporations." In reality, strict adherence to cGMP standards is even more critical for a startup. It’s your foundational proof of quality. It’s a powerful way to build immediate trust with customers and retail partners, effectively leveling the playing field with the industry's biggest players.
GMP isn't a barrier to entry for small brands; it's a strategic asset that validates your commitment to quality and protects your brand’s reputation.
Finally, some believe that GMP standards are the same everywhere. While markets like the U.S. and Europe maintain robust enforcement, global compliance can vary widely. In fact, studies have shown that in some low- and middle-income countries, a staggering 13.6% of essential medicines were found to be counterfeit or substandard. This highlights the vital public health role of strict GMP enforcement. You can learn more about the global impact of these quality standards.
Once you bust these myths, you see GMP for what it is: a non-negotiable framework for any serious brand aiming for long-term success.
Finding a True Partner in GMP Compliant Manufacturing
Choosing a manufacturer is one of the most defining decisions you'll make. This isn’t just about finding a supplier to fill bottles; it’s about finding a partner who will directly influence your product's quality, your ability to scale, and your market reputation.
A GMP certificate is the price of entry—the absolute baseline, not the finish line. A true manufacturing partner operates with transparency and collaboration, acting as an extension of your own team. They don’t just take orders; they act as your R&D collaborator from concept through launch.
Key Questions to Ask a Potential Partner
You need to cut through sales pitches and get to the heart of what a manufacturer can actually do for you. Asking the right questions is the only way to get a clear picture of their real-world capabilities around quality, flexibility, and reliability.
Here are the essential questions every brand owner should ask:
- Quality & Compliance: How do you test incoming raw materials? Can you provide a Certificate of Analysis (CoA) for every ingredient and for the finished product batch?
- Customization & Flexibility: What are your minimum order quantities (MOQs) for pilot runs versus full-scale production? How involved can our team be in the R&D and flavor development process?
- Speed & Reliability: What’s your typical turnaround time from a finalized purchase order? How do you communicate project timelines and any potential delays?
Your manufacturer should be far more than a supplier; they should be your strategic production arm. Their ability to handle pilot runs, offer R&D support, and maintain honest, open communication is just as vital as their GMP certificate.
When you find this kind of partner, you gain a dedicated team invested in your growth. They bring the operational muscle, like our advanced cold-fill technology and botanical extraction methods, so you can stay focused on building your brand. It’s this partnership mindset that turns a great product vision into a market-leading reality.
Your Top GMP Questions, Answered
We've covered the fundamentals of what GMP is and why it matters. But brand founders usually have a few more specific questions. Let's tackle the most common ones.
What's the Real Difference Between GMP and cGMP?
You’ll see both terms, and the difference is simple but critical. The 'c' in cGMP stands for current. GMP is the foundational rulebook. But cGMP means a manufacturer is actively using the most up-to-date technologies, processes, and quality systems available right now. For any competitive brand, settling for anything less than a cGMP-certified facility is a non-starter.
So, Does the FDA Hand Out GMP Certificates?
This is a huge misconception. The FDA is the enforcer—they conduct inspections and have the power to issue warnings or shut down a non-compliant manufacturer. However, the FDA does not issue GMP certificates. That stamp of approval comes from independent, third-party organizations like UL or NSF International. They perform their own demanding audits to verify a facility is truly living up to FDA standards.
Can a Single Product Be "GMP Certified"?
No. GMP certification applies to the process and the entire manufacturing facility, not an individual product. When you see a "GMP Certified" seal, it’s a promise that the product inside was made in a facility that has passed a rigorous audit. It’s a guarantee of how it was made.
How Often Do These Facilities Get Audited?
To keep their certification, manufacturers can't pass once and call it a day. Typically, third-party certifying bodies conduct audits annually. This constant oversight ensures the manufacturer maintains cGMP standards year after year, giving you peace of mind that your partner isn't cutting corners.
Ready to partner with a manufacturer that prioritizes transparency and proven quality? Triton Nutra Group is your dedicated R&D partner, offering cGMP-compliant manufacturing from pilot runs to full-scale production. Request your free quote today and let's build your next market-leading supplement together.