8 Key Quality Assurance Best Practices for 2025
Discover 8 essential quality assurance best practices for liquid supplement manufacturing. Elevate your cGMP compliance, testing, and documentation today.
 Get a Free Quote Today!
Get a Free Quote Today!Beyond the Basics: Elevating Quality in Liquid Supplement Manufacturing
In the competitive nutraceutical market, simply meeting minimum quality standards is insufficient for long-term success. True market leaders distinguish themselves through an unwavering commitment to excellence at every stage of production. This article moves beyond generic advice to provide a deep dive into specific, actionable quality assurance best practices tailored for liquid supplement manufacturing. We will explore how to implement robust systems that not only ensure cGMP compliance and regulatory adherence but also build a foundation for superior product efficacy, safety, and unwavering brand trust.
These strategies are crucial for any brand, from a garage startup to an established industry player, seeking to create a market-ready success. The following list details the essential methodologies that separate top-tier products from the rest. You will learn how to integrate advanced testing, proactive risk management, and rigorous documentation into your manufacturing process. By adopting these frameworks, you can confidently deliver supplements that meet the highest standards of safety and quality, ensuring customer satisfaction and building a reputable brand. Let's examine the key strategies that define excellence in supplement production.
1. cGMP Compliance as a Foundational Framework
Current Good Manufacturing Practices (cGMP), established by the FDA, are the bedrock of reliable supplement production. More than a simple checklist, cGMP provides a comprehensive framework for ensuring every product is consistently manufactured and controlled according to stringent quality standards. This system is one of the most vital quality assurance best practices because it builds a proactive, preventative culture rather than a reactive, problem-fixing one.
For liquid supplements, where risks like microbial growth and cross-contamination are elevated, cGMP demands rigorous control over every variable. It governs facility design, personnel hygiene, equipment calibration, and the handling of all materials from raw ingredient receipt to final product packaging. The core principle is to design and control processes to guarantee the identity, strength, quality, and purity of the final product.
Implementing cGMP in Your Operations
Adhering to cGMP means creating a controlled and documented environment. For example, a manufacturer producing a custom herbal tincture would develop a Master Batch Record (MBR). This detailed recipe dictates the precise steps, ingredients, and equipment settings for that specific formula, ensuring it is made the exact same way every time.
Another critical application is preventing cross-contamination between different liquid formulas. This involves validated cleaning procedures, such as conducting ATP swab tests on mixing tanks after cleaning to confirm no residual organic matter remains before the next batch begins. Similarly, maintaining meticulous logs for temperature-sensitive extraction equipment ensures that active botanical compounds are preserved effectively and consistently.
Actionable Tips for cGMP Success
To integrate cGMP seamlessly, consider these practical steps:
- Appoint a Dedicated Quality Manager: Designate a specific individual or team to own cGMP implementation, conduct training, and lead internal audits. This creates clear accountability.
- Digitize Documentation: Move your Standard Operating Procedures (SOPs), batch records, and training logs to a digital system. This simplifies version control, ensures immediate access for staff, and makes audit preparation far more efficient.
- Conduct Proactive Internal Audits: Don't wait for an FDA inspection. Perform regular, scheduled internal audits to identify potential compliance gaps. Use these findings to drive continuous improvement in your quality system.
- Prioritize with a Risk-Based Approach: Analyze your entire manufacturing process and identify the areas with the highest risk to product quality, such as raw material testing or fill line sanitation. Focus your initial cGMP enhancement efforts on these critical control points for the greatest impact.
2. Risk-Based Testing as a Strategic Priority
A risk-based approach to testing moves quality assurance beyond a one-size-fits-all model. Instead of testing everything with equal intensity, it strategically concentrates resources on the areas with the highest probability and impact of failure. This method is one of the most effective quality assurance best practices because it optimizes your efforts, ensuring maximum scrutiny is applied where it matters most for product safety and quality. It transforms testing from a costly, broad-spectrum activity into a focused, high-impact investment.

For liquid supplement manufacturing, this means identifying critical control points where failures would be catastrophic. For instance, the potential for microbial contamination in a finished product poses a far greater risk than a minor variation in bottle label placement. Therefore, microbiological testing of the final batch and the source water would receive more frequent and rigorous analysis than cosmetic label checks. For liquid supplement manufacturing, water purity is paramount, making rigorous testing of this key raw material essential. Consider these reliable water quality testing tips to ensure the integrity of your base ingredients.
Implementing Risk-Based Testing in Your Operations
Applying a risk-based model requires a thorough analysis of your entire production process, from raw material sourcing to final distribution. For a vitamin C liquid shot, a high-risk area is the stability of the ascorbic acid, which can degrade with exposure to heat and oxygen. A risk-based plan would prioritize accelerated stability testing to confirm shelf-life claims and implement stringent controls over mixing temperatures and bottling procedures to mitigate degradation.
Another example is focusing on potential allergens. If your facility produces both a nut-free multivitamin and a formula containing almond extract, the highest risk is cross-contact. Your testing strategy would therefore prioritize post-cleaning allergen swab tests on shared equipment. This targeted approach is more efficient and provides greater assurance of safety than randomly testing batches for a wide array of potential contaminants.
Actionable Tips for Risk-Based Success
To implement this strategic approach effectively, consider these steps:
- Create a Standardized Risk Rating System: Develop a matrix to score risks based on likelihood and severity (e.g., a 1-5 scale for each). This creates an objective framework for prioritizing testing activities and justifying resource allocation.
- Involve Cross-Functional Stakeholders: Engage personnel from production, R&D, and procurement in risk identification sessions. Their diverse perspectives are crucial for uncovering potential failure points that a single department might overlook.
- Use Historical Data to Inform Assessments: Analyze past deviations, out-of-spec results, and customer complaints. This historical data is invaluable for accurately predicting where future problems are most likely to occur.
- Document Your Risk-Based Decisions: Clearly record why certain tests are performed at a higher frequency or with greater rigor than others. This documentation is essential for regulatory audits and for demonstrating a thoughtful, systematic approach to quality control.
3. The Test Automation Pyramid
The Test Automation Pyramid is a strategic framework, popularized by experts like Mike Cohn and Martin Fowler, that guides the allocation of automation efforts. It visualizes an ideal test suite structure with a broad base of unit tests, a smaller middle layer of integration tests, and a very narrow top of UI or end-to-end tests. Adopting this model is one of the most effective quality assurance best practices because it maximizes test speed and reliability while minimizing maintenance costs and flaky results.
For software development, especially in complex systems, this approach prevents teams from over-investing in slow, brittle UI tests. Instead, it prioritizes fast, isolated unit tests that provide immediate feedback to developers, catching bugs at the earliest and cheapest stage. The pyramid’s structure directly addresses the common pitfall of an inverted pyramid, or "ice-cream cone" anti-pattern, where teams rely too heavily on slow, expensive end-to-end tests, leading to long feedback cycles and an unstable testing process.
This infographic visualizes the Test Automation Pyramid, illustrating the recommended proportional distribution across the three primary test layers.
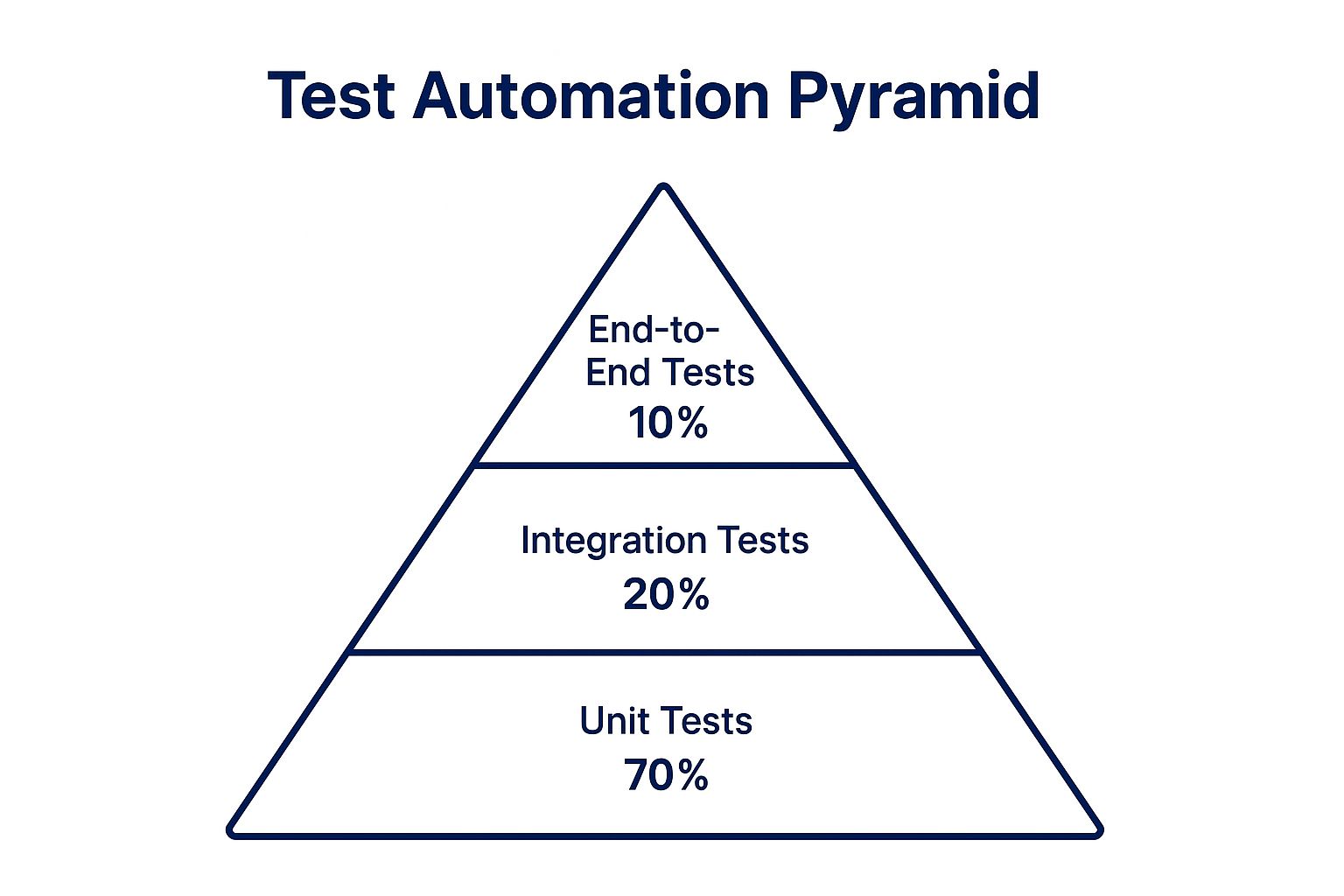
The visual hierarchy clearly shows that the vast majority of automated tests should be fast, low-level unit tests, forming a stable foundation for the entire quality strategy.
Implementing the Test Automation Pyramid
Adopting the pyramid means deliberately shifting your testing focus to the lower levels. For instance, a development team at a company like Google might follow a 70-20-10 ratio, dedicating 70% of their automation efforts to unit tests that verify individual functions or classes in isolation. These tests run in milliseconds, allowing for continuous execution with every code change.
The middle layer involves integration tests, which verify interactions between different components or microservices. A key technique here is contract testing, where services agree on an "API contract." This ensures that even when developed independently, they can communicate reliably without requiring a fully deployed environment for every test. The final 10% is reserved for end-to-end UI tests, which validate critical user journeys, such as the complete checkout process on an e-commerce site, ensuring all integrated parts work together as expected from a user’s perspective.
Actionable Tips for Pyramid Success
To build a robust and efficient test suite using this model, consider these steps:
- Establish a Coverage Target: Aim for high unit test coverage (e.g., 80% or more) for new code. Use code coverage tools to measure this and make it a visible part of your team's definition of "done."
- Leverage Service Virtualization: For integration tests, use tools that simulate the behavior of external services or APIs. This isolates the component under test and avoids dependencies on unstable external systems.
- Focus UI Tests on Critical Paths: Resist the temptation to automate every possible user interaction through the UI. Limit these tests to the most critical, high-value user workflows to reduce flakiness and maintenance overhead.
- Integrate into CI/CD Pipelines: Ensure your unit and integration tests run automatically with every code commit. This provides rapid feedback and enforces quality gates, preventing faulty code from progressing to production.
4. Continuous Integration and Continuous Testing
While often associated with software development, the principles of Continuous Integration (CI) and Continuous Testing (CT) offer a powerful model for modern quality assurance. This approach involves automating the process of validating changes to ensure they meet predefined quality standards. Adopting this mindset is one of the most forward-thinking quality assurance best practices because it shifts testing from a final, separate stage to an integrated, ongoing activity that provides immediate feedback.
In a manufacturing context, this translates to systems that continuously monitor and validate processes. Instead of waiting for a batch to be completed before testing, you integrate automated checks throughout the production line. Imagine an in-line sensor that constantly measures the pH or viscosity of a liquid supplement as it’s being mixed, automatically flagging any deviation from the Master Batch Record in real-time. This prevents an entire batch from being compromised, saving significant time and resources.
Implementing Continuous Methodologies in Your Operations
Applying CI/CT principles means embedding automated quality checks directly into your workflow. For instance, a manufacturer could use a Manufacturing Execution System (MES) that integrates with production equipment. As a batch of a vitamin C liquid is produced, the system automatically logs data from scales, mixers, and temperature probes. If a weight measurement for a key ingredient falls outside the specified tolerance, the system can halt the process and alert an operator immediately, preventing a costly error before it escalates.
Another example is leveraging automated visual inspection systems on a bottling line. These systems use high-speed cameras and machine learning to check for proper fill levels, cap sealing integrity, and label placement on every single bottle. This provides a 100% inspection rate that is far more reliable and faster than manual spot-checking, ensuring every unit meets quality specifications before it is packed.
Actionable Tips for Continuous Quality Success
To integrate these continuous principles effectively, consider these practical steps:
- Start with a Simple Automated Check: Begin by automating a single, high-impact quality check. This could be an in-line temperature monitor or an automated scale that logs data directly to your batch record. Build on this initial success.
- Implement Fast-Failing Tests First: Prioritize automated checks at the earliest stages of production, such as raw material verification. Catching an issue here is far less costly than discovering it in the final product.
- Use Parallel Data Monitoring: Instead of reviewing data sequentially, set up systems to monitor multiple critical control points simultaneously. A central dashboard showing real-time metrics from mixing, filling, and packaging provides a holistic view of quality.
- Establish a Robust Alert System: Ensure your automated systems are configured to send immediate, clear alerts to the right personnel when a deviation occurs. This enables rapid intervention and minimizes production downtime.
5. Adopting Test-Driven Development (TDD) for System Integrity
While seemingly a software-specific discipline, Test-Driven Development (TDD) offers a powerful mental model and procedural blueprint for any system requiring high reliability, including the digital systems that manage manufacturing. Popularized by pioneers like Kent Beck, TDD flips the traditional development cycle on its head. Instead of writing code first and testing it later, you write a failing test before you write any functional code. This methodology is one of the most effective quality assurance best practices because it forces clarity, precision, and a focus on requirements from the very beginning, ensuring that every component functions exactly as intended.
The TDD process follows a simple, repeating cycle: Red, Green, Refactor. First, you write an automated test for a new feature (Red, because it will fail). Next, you write the absolute minimum amount of code required to make that test pass (Green). Finally, you clean up and improve the code's structure (Refactor) without changing its external behavior, ensuring all tests remain green. This embeds quality directly into the development process, rather than treating it as a final inspection step.
Implementing TDD in Your Quality Systems
Applying a TDD mindset is crucial for the software that underpins modern manufacturing, such as your Quality Management System (QMS) or inventory tracking software. For example, before developing a new feature to automatically quarantine raw materials that fail specification tests, a developer would first write a test that simulates a failing batch and asserts that the system correctly assigns it a "quarantined" status. Only then would they write the code to make that quarantine logic work.
This approach prevents critical errors. Imagine a feature meant to alert staff when a temperature-controlled storage unit deviates from its set range. Using TDD, a test is first created to confirm an alert is triggered under these specific fault conditions. This ensures the alerting mechanism, a critical control point, is verifiably functional before it is even built, preventing silent failures that could lead to spoiled ingredients and compromised product safety.
Actionable Tips for TDD Success
To integrate a TDD approach effectively, consider these steps:
- Start with Small, Clear Requirements: Begin by applying TDD to simple, well-defined features, such as validating user input on a data entry form. Success here builds confidence and momentum for more complex tasks.
- Write the Minimum Code to Pass: Resist the urge to write complex code or add extra features. The goal is only to satisfy the immediate test. This keeps the process focused and the codebase lean.
- Refactor After Every Green Light: Make refactoring a non-negotiable step. After each test passes, look for opportunities to improve clarity, remove duplication, and simplify the design. This discipline prevents technical debt.
- Use Descriptive Test Names: Name your tests clearly to describe their intent and expected outcome (e.g.,
testAlertsTriggeredWhenFreezerTempExceedsLimit). This turns your test suite into living documentation for your system's behavior.
6. Exploratory Testing
Exploratory testing is a dynamic approach that leverages the tester's freedom, curiosity, and expertise to learn about the software, design tests, and execute them simultaneously. Unlike scripted testing, which follows a predefined path, this method is an unscripted, real-time investigation. It is one of the most effective quality assurance best practices for discovering unexpected defects, usability issues, and edge cases that rigid test plans often miss, treating the testing process as a cognitive activity rather than a simple clerical task.
Popularized by QA experts like James Bach and Cem Kaner, this approach empowers testers to act like detectives, following clues and adapting their strategy based on what they find. For example, a tester for a new feature on Slack might start by following the expected user flow but then deviate by inputting unusual character sets, rapidly switching between channels, or testing integrations in unconventional ways. This free-form investigation reveals bugs and user experience flaws that a structured test case would not have anticipated.
Implementing Exploratory Testing in Your Operations
Successfully integrating exploratory testing requires structure, even within its unscripted nature. One effective method is Session-Based Test Management (SBTM). Here, a tester is assigned a specific "charter" or mission for a time-boxed session, such as "Investigate the user profile update functionality for 90 minutes and identify potential data validation errors."
During the session, the tester documents their path, notes any discovered bugs, and records ideas for future tests. For instance, a game tester at a company like Electronic Arts might explore a new game level with the charter of finding graphical glitches. As they play, they might notice a texture failing to load when a specific sequence of actions is performed, a discovery made through organic interaction rather than a step-by-step script. This documentation provides valuable insights for developers and informs future testing cycles.
Actionable Tips for Exploratory Testing Success
To maximize the value of exploratory testing, consider these practical steps:
- Time-Box Exploration Sessions: Set clear time limits (e.g., 60-120 minutes) for each testing session. This creates focus, prevents aimless wandering, and ensures testers cover their assigned charter efficiently.
- Use a Risk-Based Approach: Prioritize exploratory efforts by focusing on the most complex, high-risk, or recently changed areas of the software. This ensures that your most creative testing resources are applied where they can have the greatest impact.
- Document Findings Immediately: Encourage testers to use simple tools (even just a text file or spreadsheet) to log their actions, observations, questions, and bug reports as they happen. This "note-taking" is crucial for reproducibility and sharing insights.
- Pair Testers for Collaboration: Conduct paired exploratory sessions where two testers work together at one computer. One person navigates the software while the other asks questions and takes notes, fostering a richer, more critical analysis.
7. Quality Gates and Entry/Exit Criteria
Quality gates are predefined checkpoints within the software development lifecycle where specific, measurable quality criteria must be met before a project can proceed to the next phase. This structured approach prevents defects from moving downstream, saving significant time and resources. Implementing formal gates is one of the most effective quality assurance best practices because it transforms quality from a final inspection activity into a continuous, integrated part of the entire development process.

This methodology forces teams to pause and verify that standards for code quality, security, performance, and documentation are satisfied. For example, before a build can move from development to a staging environment, an automated quality gate might check that unit test coverage exceeds 85%, no new critical security vulnerabilities have been introduced, and all code has been successfully peer-reviewed. This ensures that only stable, secure, and well-vetted code advances, reducing the likelihood of major issues during later testing stages.
Implementing Quality Gates in Your Operations
Integrating quality gates requires defining clear entry and exit criteria for each development stage. A powerful real-world example is in medical device software, where FDA validation is mandatory. A gate between the development and validation phases might require documented evidence that all software requirements have been traced to specific tests and that risk analysis for each feature has been completed and signed off. This prevents the costly scenario of discovering a major compliance gap just before a planned product release.
In another scenario, a financial technology company might establish a pre-production gate to enforce regulatory compliance. The exit criteria could include a successful penetration test report from a third-party security firm and confirmation that all data handling routines comply with PCI DSS standards. If these criteria are not met, the release is automatically halted, preventing potentially catastrophic compliance violations and data breaches.
Actionable Tips for Quality Gate Success
To effectively implement quality gates, consider these practical steps:
- Define Clear, Measurable Criteria: Avoid vague goals like "good code quality." Instead, use specific metrics such as "code complexity score below 15" or "zero high-severity static analysis warnings."
- Align Gates with Business Objectives: Ensure your quality gates support key business outcomes, such as faster time-to-market or enhanced security. A gate focused on performance testing is critical for a high-traffic e-commerce platform.
- Automate Gate Checks Where Possible: Integrate your gates into your CI/CD pipeline using tools like Jenkins, GitLab CI, or SonarQube. This provides instant feedback and removes the potential for human error or bias.
- Regularly Review and Update Criteria: As your product, technology stack, and business goals evolve, your quality standards must also adapt. Review and adjust gate criteria quarterly or after major project milestones to ensure they remain relevant and effective.
8. Defect Prevention and Root Cause Analysis
A mature quality system moves beyond simply catching mistakes to actively preventing them. This proactive stance is embodied by defect prevention and root cause analysis, a cornerstone of quality assurance best practices. Instead of just inspecting and rejecting a bad batch of liquid supplements, this methodology digs deeper to understand why the defect occurred and implements systemic changes to stop it from happening again. It shifts the focus from a costly reactive cycle to a value-driven preventative one.
This approach, popularized by quality pioneers like W. Edwards Deming, is essential for liquid supplement manufacturing where issues like inconsistent viscosity, incorrect ingredient concentration, or microbial contamination can have significant safety and financial implications. The goal is to build quality into the process itself, making it difficult, if not impossible, for defects to occur in the first place. This philosophy aligns with the "Zero Defects" concept, striving for perfection by addressing problems at their source.
Implementing Defect Prevention in Your Operations
Applying this practice means creating a culture of inquiry and continuous improvement. For instance, if a batch of vitamin D3 drops is found to have a lower-than-specified potency, a simple correction is to discard the batch. A root cause analysis approach, however, would investigate further. Was the raw material improperly stored? Was the mixing equipment calibrated incorrectly? Did a specific operator deviate from the Standard Operating Procedure (SOP)?
By using a structured method like the "5 Whys," the team might discover the root cause was an uncalibrated scale used for weighing the D3 concentrate. The corrective action is to calibrate the scale, but the preventive action is to implement a mandatory, logged calibration check before every single batch. This systemic change prevents the issue from ever recurring for that reason, protecting future production runs across all product lines.
Actionable Tips for Defect Prevention Success
To embed this proactive mindset into your quality system, follow these steps:
- Use Structured Root Cause Analysis (RCA) Methods: Train your quality team on formal RCA techniques like the 5 Whys or Fishbone (Ishikawa) diagrams. Use these tools to guide investigations and avoid jumping to superficial conclusions.
- Implement Defect Tracking and Trending: Log every single deviation, non-conformance, and defect in a centralized system. Regularly analyze this data to identify patterns, such as a specific machine causing frequent issues or a particular process step being a common failure point.
- Focus on Process Improvements: The output of any root cause analysis should be a tangible change to a process, SOP, or training module. Prioritize improvements that offer the highest impact on preventing defect recurrence.
- Create Defect Prevention Checklists: For critical process steps like formulation mixing or bottling, develop specific checklists that operators must complete. These act as real-time verification tools to ensure known failure points are checked and controlled during production.
Quality Assurance Best Practices Comparison
| Approach | Implementation Complexity | Resource Requirements | Expected Outcomes | Ideal Use Cases | Key Advantages |
|---|---|---|---|---|---|
| Shift-Left Testing | Moderate - requires cultural/process changes | Skilled testers early, tools, training | Early defect detection, shorter cycles | Development phases, Agile/DevOps teams | Reduces defect fix costs, enhances quality |
| Risk-Based Testing | High - requires upfront risk analysis | Risk assessment skills, stakeholder input | Focused testing on critical areas | Business-critical applications | Optimizes resources, reduces project risk |
| Test Automation Pyramid | Moderate to high - layered test development | Automation skills across layers | Fast feedback, maintainable tests | Projects needing scalable test automation | Cost-effective, scalable, faster feedback |
| Continuous Integration & Testing | High - infrastructure and automation setup | Automation tools, infrastructure | Early integration issue detection, faster release | Frequent code changes and deployments | Improves code quality, speeds releases |
| Test-Driven Development (TDD) | Moderate - requires developer discipline | Skilled developers, time for tests | High test coverage, better code design | Code development requiring reliability | Ensures quality, reduces debugging time |
| Exploratory Testing | Low to moderate - relies on tester expertise | Skilled testers | Finds unexpected issues, usability insights | Agile teams, dynamic or changing software | Adaptable, uncovers hidden defects |
| Quality Gates and Entry/Exit Criteria | Moderate - needs clear criteria definition | Process management, stakeholder involvement | Consistent quality checkpoints | Regulated environments, phase-based projects | Prevents defect propagation, clear milestones |
| Defect Prevention & Root Cause Analysis | Moderate to high - requires analysis and process change | Analytical tools, team commitment | Reduced defect rates, improved processes | Organizations focused on quality improvement | Prevents recurrence, improves efficiency |
Integrating Quality Assurance as Your Competitive Advantage
The journey through these eight foundational quality assurance best practices reveals a powerful truth: true quality is not an afterthought or a final checkpoint. It is a proactive, deeply integrated discipline that serves as the bedrock of a successful liquid supplement brand. From the early-stage foresight of Shift-Left Testing to the meticulous precision of Test-Driven Development (TDD), each practice is a vital component in a larger system designed to build excellence into every drop of your product.
Moving beyond basic compliance, these strategies transform your operations. Embracing a Test Automation Pyramid and Continuous Integration/Continuous Testing (CI/CT) pipelines doesn't just speed up production; it creates a resilient, agile framework capable of adapting to market demands and regulatory shifts. Meanwhile, applying Risk-Based Testing ensures your resources are focused where they matter most, protecting your brand against the most significant threats to product integrity and consumer safety. This strategic allocation of effort is what separates industry leaders from the rest.
From Principles to Actionable Strategy
The core takeaway is that implementing these quality assurance best practices is a strategic business decision, not merely a technical one. The real value emerges when these principles are woven into your company's DNA. This means empowering your teams with the mandate for Exploratory Testing, encouraging them to think like a critical consumer, and rigorously enforcing Quality Gates to prevent defects from ever reaching the next stage. It involves a steadfast commitment to Defect Prevention and Root Cause Analysis, ensuring that every issue becomes a lesson that strengthens your entire process for the future.
This continuous cycle of improvement is the engine of brand trust. For those looking to deepen their understanding of QA principles and workflows, exploring resources on mastering quality assurance workflows and techniques can provide valuable insights into building these robust systems. Ultimately, this dedication to quality creates a powerful competitive advantage. It builds a reputation for reliability and efficacy that resonates with consumers, satisfies regulatory bodies, and secures your position as a market leader.
Your Path to Market-Leading Quality
Adopting these advanced QA methodologies is a significant undertaking, but the rewards are immense. It's about building more than a compliant product; it's about creating a legacy of trust and excellence. By embedding these quality assurance best practices into your operational philosophy, you aren't just meeting standards. You are setting them. This commitment is the definitive hallmark of a brand that customers will not only choose but will champion for years to come.
Ready to partner with a manufacturer that lives and breathes these principles? Triton Nutra Group builds every one of these quality assurance best practices into our liquid supplement manufacturing process to guarantee product safety, efficacy, and compliance. Contact Triton Nutra Group today to see how our commitment to quality can become your brand's greatest asset.