GMP Supplement Certification: The Ultimate Guide for Brand Founders
Your guide to GMP supplement certification. Learn how GMP compliance builds brand trust, ensures quality, and helps you choose the right manufacturing partner.
 Get a Free Quote Today!
Get a Free Quote Today!GMP supplement certification is your brand's ultimate seal of quality. It’s a comprehensive system of processes, procedures, and meticulous documentation ensuring your dietary supplements are consistently produced and controlled according to the highest standards. This third-party verification, typically from recognized bodies like UL or NSF, confirms that your manufacturing partner adheres to the FDA's Current Good Manufacturing Practices (cGMP). In simple terms, it guarantees what’s on the label is exactly what’s in the bottle—nothing more, nothing less.
What GMP Certification Really Means for Your Brand
Think of GMP certification not as a finish line, but as the master blueprint for a world-class manufacturing operation. It’s the rulebook dictating every single action—from how raw ingredients are received and inspected to the precise temperature of a botanical extraction and the final tamper-proof seal on the bottle.
For you as a brand founder, this isn't just a certificate to hang on the wall; it’s your ultimate brand protection.
This system is designed to prevent the kinds of errors that could cripple your business before you even know they’ve happened. It’s what stops cross-contamination, formula mix-ups, and potency deviations in their tracks. A cGMP-certified partner operates on a core principle: "If it wasn't written down, it didn't happen." This meticulous documentation creates an unbreakable chain of custody for your product, ensuring total accountability from concept to launch.
The Core Pillars of GMP in Practice
At its heart, GMP is built on a few non-negotiable pillars that directly impact the quality and integrity of your finished supplement. Understanding them helps you see the true value your manufacturing partner builds into every single unit.
- Personnel: Everyone involved in manufacturing must be expertly trained for their specific role. This ensures they understand our strict Standard Operating Procedures (SOPs), hygiene requirements, and the importance of following them without fail.
- Premises & Equipment: The facility itself is designed to prevent contamination. This means logical workflows, advanced air filtration, and surfaces that are easy to clean and sanitize. All equipment is calibrated, cleaned, and maintained on a strict schedule to ensure peak performance.
- Processes & Controls: Every step, from creating your custom formula to bottling the final product, is governed by a Master Manufacturing Record (MMR). This is the secret to consistency, ensuring every batch is made the exact same way.
- Raw Materials: A robust cGMP system includes rigorous vendor qualification and incoming raw material testing. No ingredient enters our production line until it has been quarantined, tested, and verified for identity, purity, and potency.
"A commitment to cGMP (Current Good Manufacturing Practices) is a commitment to continuous improvement. It means your manufacturing partner is actively using up-to-date technologies and systems, ensuring your product isn’t just compliant with yesterday’s standards, but is built for today’s market demands."
Beyond the Certificate: The Partnership Mindset
Achieving and maintaining cGMP certification is an intensive, ongoing process. It requires deep investment in infrastructure, technology, and team training. When you partner with a manufacturer that holds these credentials, you're not just buying a product; you are leveraging our entire commitment to quality. This frees you up to focus on what you do best: building your brand and connecting with your customers.
This level of operational discipline is crucial. A strong cGMP framework is the foundation for everything we do, from our innovative cold-fill technology that preserves delicate botanicals to our proprietary extraction methods. It ensures that every innovation is built upon a reliable and repeatable system. To go deeper, our detailed guide on supplement quality control explores the many layers of testing that protect your product.
While our focus is on supplements, it’s insightful to see how GMP fits into the broader regulatory world, like the rigorous FDA approval process for medical devices. Both industries share a foundational commitment to safety, documentation, and process validation. This parallel really underscores why choosing a partner with a deep-seated culture of compliance is such a powerful strategic advantage.
Why GMP Compliance Is Your Greatest Competitive Advantage
In a crowded market, a clever formula or slick branding can only get you so far. Real, lasting success is built on a foundation of trust—and in the supplement world, GMP certification is the absolute cornerstone of that foundation. It’s a strategic move that turns a regulatory requirement into one of your most powerful marketing assets.
Partnering with a cGMP-certified manufacturer isn't about ticking a box. It’s about future-proofing your brand. This is your single best defense against the operational and reputational disasters that can derail a growing business, from inconsistent product batches to a catastrophic recall.
Think of it as the ultimate insurance policy. A manufacturer truly committed to cGMP doesn't just make supplements; they produce consistency, reliability, and peace of mind. This frees you up to focus on what you do best—marketing and growing your brand—confident that the product in every single bottle is safe, pure, and exactly what the label says it is.
Unlocking Premier Retail and E-commerce Channels
Having an amazing product is pointless if your ideal customers can't buy it. Today, major retailers and e-commerce giants like Amazon act as strict gatekeepers, and their number one priority is eliminating risk. For them, a supplement brand without verifiable GMP credentials is a complete non-starter.
Trying to get onto these platforms without proof of cGMP compliance is like showing up to the airport without an ID. The doors simply will not open.
- Amazon FBA: Demands extensive documentation proving your products are made in a cGMP-compliant facility just to get a listing approved.
- Major Retail Chains: Big players like Walmart, Whole Foods, and GNC have their own tough quality standards, and GMP is the absolute minimum requirement to even start the conversation.
- International Markets: Thinking about going global? cGMP is the universally recognized benchmark for quality, making it a prerequisite for expansion.
When you work with a cGMP-certified partner like us, you show up on day one with all the right paperwork in hand. We deliver the comprehensive documentation and compliance support you need for a smooth entry into the most profitable sales channels, making sure red tape doesn't kill your launch momentum.
Building Unbreakable Consumer Trust
Today’s customers are savvier and more skeptical than ever before. They research ingredients, double-check labels, and actively hunt for brands that show a real commitment to safety and transparency. A GMP seal on your product acts as an instant, powerful signal of trust that cuts right through the noise.
It immediately tells your customer, "We don't cut corners. We invested in the highest quality processes to make sure the product you're holding is safe and effective." You can't put a price on that message of integrity.
In an industry where trust is everything, cGMP compliance isn’t just a feature—it’s the entire foundation of your brand's relationship with its customers. It shows you prioritize their well-being above all else, creating the kind of loyalty that turns one-time buyers into lifelong fans.
This trust pays off directly with higher conversion rates, better customer retention, and a stronger reputation that protects you from low-quality competitors. It’s a clear point of difference that smart consumers look for and reward. This trend is only getting stronger globally as organizations work to improve safety across the supply chain. For instance, key players like NSF International now offer certifications like NSF/ANSI 455-2 for dietary supplements, which involves rigorous audits based on both regulatory rules and industry best practices. Earning this allows companies to use globally recognized logos, giving their brand credibility a massive boost. You can learn more about how NSF is raising the bar for the industry on NSF.org.
Turning Compliance into a Marketing Superpower
At the end of the day, partnering with a manufacturer that lives and breathes the strictest cGMP standards is more than just a compliance strategy—it's a growth strategy. It gives you the power to stand behind your product with total confidence, knowing it’s backed by a system of unmatched quality control.
That confidence becomes a central part of your brand’s story. You can proudly feature your commitment to quality in your marketing campaigns, on your website, and all over your packaging. It becomes a key selling point that positions your brand as a premium, trustworthy choice in a sea of uncertainty, giving you a distinct and sustainable edge over the competition.
A Look Inside a GMP Certified Manufacturing Process
Ever wonder what really happens behind the scenes at a top-tier manufacturing facility? A GMP supplement certification isn't just a piece of paper. It’s a living, breathing system that dictates every single action, from the moment a raw botanical arrives at our loading dock to the final quality check before your product ships.
Pulling back the curtain reveals a process built on precision, documentation, and an unwavering commitment to quality.
For you, understanding this process is crucial. It’s the difference between blindly trusting a partner and confidently knowing your product is being made right—every single time. This inside look empowers you to ask smarter questions and identify a manufacturer who doesn't just meet standards but actually sets them.
This infographic breaks down how a robust GMP system translates into tangible benefits for your brand, from risk management to building unbreakable consumer trust.
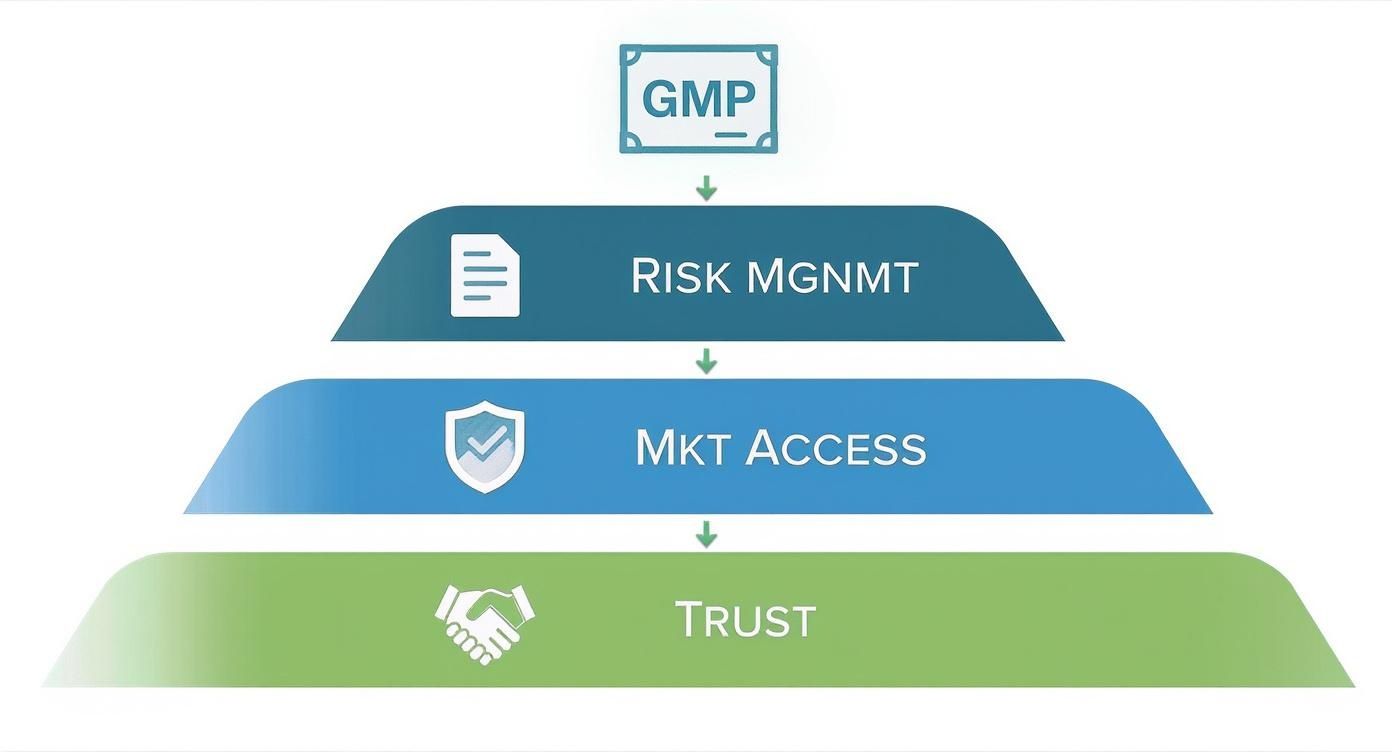
As you can see, cGMP compliance is the foundation that supports critical business advantages like accessing new markets and mitigating risk, all of which culminate in enhanced brand trust.
From Quarantine to Production: The Raw Material Journey
The integrity of your final product begins long before any blending or bottling ever occurs. It starts with an uncompromising approach to raw materials. At a cGMP-certified facility, no ingredient is ever taken at face value.
The process kicks off with a strict vendor qualification protocol. We don't just find a supplier; we validate them through a deep dive into their own quality systems and testing procedures. Only after they pass this rigorous vetting do they become an approved partner.
Once a shipment arrives, it doesn't go straight to the warehouse floor. Instead, it enters a designated quarantine area. Here, our quality control team pulls samples for a battery of identity, purity, and potency tests. The material remains locked down until our in-house lab confirms it meets every single specification. This "trust but verify" philosophy is non-negotiable.
The Master Manufacturing Record: The Blueprint for Consistency
How do you guarantee that batch #100 is absolutely identical to batch #1? The answer lies in the Master Manufacturing Record (MMR). Think of the MMR as the master recipe for your product, detailing every single step with painstaking precision.
"A core tenet of GMP is: 'If it wasn't written down, it didn't happen.' The MMR is the ultimate manifestation of this principle. It is the single source of truth that ensures absolute repeatability, batch after batch, year after year."
The MMR isn't just a list of ingredients. It’s a comprehensive document that specifies:
- Exact quantities of every component, down to the milligram.
- Specific equipment to be used, from the blender model to the bottling line.
- Detailed instructions and procedural checkpoints for every stage.
- Quality control checks that must be performed and signed off on during production.
Every time we produce your supplement, we generate a unique Batch Production Record (BPR) from the MMR. Our operators follow it to the letter, documenting every action in real time. This creates a complete, traceable history for every single bottle that leaves our facility.
People and Premises: The Environment of Quality
A perfect formula can be completely compromised by a flawed environment or untrained personnel. GMP addresses this head-on with strict rules governing both the facility and the people working within it.
The table below breaks down the key pillars that turn a building into a true cGMP-compliant facility, covering everything from the physical layout to the expert staff.
Key Pillars of cGMP Compliance in Supplement Manufacturing
| GMP Pillar | Key Requirements & Activities |
|---|---|
| Personnel | Continuous training on SOPs, hygiene protocols, and cGMP regulations. Clear responsibilities and qualifications for all roles. |
| Premises & Facility | Controlled workflows to prevent cross-contamination. Advanced air filtration systems and easily sanitized surfaces. Designated zones for quarantine, production, and storage. |
| Equipment | Regular calibration and maintenance schedules to ensure consistent performance. Thorough cleaning and sanitization protocols. Equipment logs to track usage and maintenance history. |
| Raw Materials | Strict vendor qualification and approval process. Quarantine and testing of all incoming materials for identity, purity, and potency before release. |
| Production Controls | Use of Master Manufacturing Records (MMRs) and Batch Production Records (BPRs) to ensure consistency. In-process quality checks and verifications. |
| Documentation | Meticulous record-keeping for all activities. "If it wasn't written down, it didn't happen" philosophy. Complete traceability from raw material to finished product. |
Each of these pillars is interconnected, creating a holistic system where quality is built into every step.
Our facility is designed with controlled workflows to prevent cross-contamination. This includes dedicated zones for different production stages and surfaces that are easily sanitized. Equipment isn't just wiped down; it's calibrated and maintained on a meticulous schedule to ensure it always performs exactly as intended.
Furthermore, our team undergoes continuous training on Standard Operating Procedures (SOPs) and hygiene protocols. They aren't just workers; they are highly trained technicians who understand their critical role in protecting your product's integrity. To fully appreciate a cGMP certified manufacturing process, it's helpful to also consider broader essential quality assurance best practices that ensure product integrity.
This commitment to both process and people is what turns cGMP from a set of rules into a true culture of quality.
The Strategic Roadmap to Achieving GMP Certification
Getting a GMP supplement certification isn’t a one-time event. Think of it as a deep, ongoing commitment to excellence. For you as a founder, understanding this journey is key to appreciating the immense value a certified partner brings to the table. This roadmap isn’t just about passing a test—it's about building an unbreakable culture of quality from the ground up.
Trying to navigate this path alone is a monumental task, packed with complexities that can drain your resources and stall your growth. When you partner with a manufacturer who has already mastered this process, you inherit a robust, battle-tested quality system. This saves you years of effort and lets you focus on what you do best: building your brand.
Stage 1: Gap Analysis and System Development
The journey kicks off with an honest, exhaustive gap analysis. This is like a complete diagnostic check-up for the entire facility. Quality experts scrutinize every single process, from how raw materials are received to how final products are shipped, identifying any area that falls short of cGMP standards.
This initial review is critical because it lays the foundation for the entire quality system. It’s all about pinpointing weaknesses before they can become liabilities.
Once the analysis is complete, the next step is developing hundreds of pages of Standard Operating Procedures (SOPs). These aren’t vague guidelines; they are meticulously detailed, step-by-step instructions for every single task performed in the facility. Think of them as the operating system for quality, ensuring every action is performed consistently and correctly, every single time.
Stage 2: Implementation and Internal Audits
With a full suite of SOPs in hand, the next phase is all about implementation and training. This is where the plans on paper become real-world actions. Every team member, from the receiving dock to the production line, goes through intensive training on the new procedures until they become second nature.
To prepare for the final audit, a top-tier manufacturer will conduct rigorous internal audits. These are dress rehearsals designed to be even tougher than the real thing.
A proactive internal audit is the sign of a mature quality culture. It’s about finding and fixing your own issues before an external auditor does. This shows a true commitment to continuous improvement, not just a desire to pass a test.
This stage is crucial for fine-tuning processes and making sure the entire team is operating in perfect sync with cGMP requirements. It’s a period of intense focus where the quality management system is tested, stressed, and ultimately perfected.
Stage 3: The Third-Party Certification Audit
The final hurdle is the third-party audit from a recognized body like UL or NSF. An independent auditor arrives on-site for a multi-day inspection, going through every aspect of the operation with a fine-tooth comb. They review documentation, observe processes in action, interview staff, and inspect the facility from top to bottom to verify that the implemented systems meet every single cGMP regulation.
Passing this audit is the ultimate validation of a manufacturer's commitment to quality. The regulatory landscape here has evolved significantly. Since June 2007, the FDA has enforced specific cGMPs for all dietary supplement manufacturers, holding them to standards similar to those for pharmaceuticals. While certification itself is technically voluntary, it's the undisputed gold standard for proving compliance. You can read more about this evolution at Drug Target Review.
This entire process hammers home why a partnership approach is so powerful. By choosing a certified manufacturer, you sidestep this whole complex and demanding journey. For a deeper dive into what's required, check out our guide on dietary supplement manufacturing requirements. You get to launch with total confidence, backed by a partner whose operational excellence is already verified.
How to Choose the Right GMP Certified Partner

Picking a manufacturing partner is one of the biggest calls you'll make. A GMP supplement certification isn't just a nice-to-have; it's the absolute minimum entry ticket. But that certificate is just the beginning of the story.
While the paperwork proves they meet the baseline, it won’t tell you if they’re agile, transparent, or genuinely collaborative. You need to dig deeper to find a partner who will act as an extension of your own team.
Your real goal is to find a manufacturer whose quality culture is so ingrained that they’re spotting potential issues before they become problems, solving them proactively, and truly caring about your brand's success. Think of them as your R&D partner, collaborating with you from concept through launch.
Asking the Right Questions Beyond the Certificate
Any manufacturer can wave a certificate in your face. A great partner, however, can confidently answer the tough questions. When you're vetting potential partners, come armed with this list to get a real sense of how they operate day-to-day.
- Audit History and Findings: "Could you walk me through your last third-party audit? What were the main findings, and how did you address them?" An open, transparent partner is a good sign. Any hesitation is a huge red flag.
- Traceability Systems: "Let's say a single lot of a raw ingredient gets recalled. Show me your process. How fast can you track down every single finished product that contains it?" You're looking for a quick, precise answer that shows their systems are airtight.
- Out-of-Spec (OOS) Protocol: "What happens when a batch—either raw material or in-process—fails a quality test? What's the exact procedure?" Don't settle for "we just reject it." A solid partner will have a detailed, documented process involving quarantine, investigation, and clear communication with you.
These questions cut through the fluff and reveal the real strength of their quality systems and their commitment to transparency. A manufacturer that welcomes this level of scrutiny is one you can build a lasting business with. For a deeper dive into that first line of defense, check out our guide on the importance of supplement ingredient testing.
Green Flags Identifying a True Manufacturing Partner
While you're on the lookout for red flags, it's just as crucial to spot the positive signs—the "green flags"—that show you've found a high-caliber partner who's ready to help you grow.
A partnership mindset changes everything. It means your manufacturer isn't just a vendor filling orders; they’re a strategic asset offering insights on formulation, compliance, and how to scale, all to help your brand dominate the market.
Keep an eye out for these indicators of a quality-driven, forward-thinking operation:
- Advanced Certifications: Do they hold certifications that go beyond the GMP baseline, like UL, NSF, or organic? This shows they’re committed to exceeding requirements and have invested in specialized quality systems.
- Flexible Run Sizes: Can they handle a small pilot batch for a new product launch just as expertly as a massive production run, all without a drop in quality? This flexibility to scale from pilot to full production is essential for startups and growing brands.
- Proactive Communication: Do they give you clear, transparent timelines and keep you in the loop with regular updates? A true partner ensures you’re never left in the dark, delivering on time and on budget.
These green flags are hallmarks of a manufacturer that isn't just compliant, but is built for partnership and growth. This level of investment in quality often comes from more established companies. In fact, research indicates that factors like company size and the number of product categories they handle positively correlate with GMP adoption, suggesting these firms are better primed for a true partnership. You can discover more insights from this study on GMP adoption.
Choosing the right manufacturer is about finding a shared commitment to excellence. When you find one who can nail the tough questions and waves all these green flags, you’re not just buying a service; you're investing in a relationship that will become your brand’s greatest asset.
Your Top Questions About GMP Supplement Certification, Answered
As a brand founder, navigating the world of manufacturing can feel like a maze of regulations and acronyms. We get it. That’s why we’re tackling some of the most common questions we hear about GMP supplement certification. Our goal is to cut through the noise and show you how partnering with the right manufacturer isn't a cost, but a powerful strategic advantage that turns compliance into a catalyst for growth.
What Is the Difference Between GMP and cGMP?
This is a fantastic question, and the answer tells you a lot about a manufacturer's philosophy. GMP stands for Good Manufacturing Practices—the basic quality standards. But that little 'c' in cGMP? That's the real game-changer.
The 'c' stands for Current. It means your manufacturing partner isn't just following a static set of rules; they are committed to using up-to-date technologies and systems that align with today’s best practices. It signals a deep commitment to continuous improvement and innovation.
When a partner is dedicated to cGMP, you know they have a proactive mindset. They’re constantly refining their processes and investing in new tech, like our advanced cold-fill systems and botanical extraction methods, to guarantee your product gets the absolute best in safety, purity, and efficiency. This forward-thinking approach is what separates the good partners from the great ones.
Is GMP Certification Required by Law?
This is a major point of confusion, so let's clear it up. The FDA legally requires every supplement manufacturer to follow cGMP regulations, as outlined in 21 CFR Part 111. This isn't optional—it's mandatory.
However, the FDA doesn't actually hand out "GMP certificates." The certification itself is a voluntary process where an independent, third-party organization like UL or NSF performs a deep-dive audit to verify that a facility is, in fact, following all those regulations to the letter.
Think of it like this: FDA compliance is the law of the road you have to follow. The third-party certification is the official inspection sticker on your windshield, proving to everyone—including major retailers and discerning customers—that your vehicle is in perfect working order.
Will GMP Processes Slow Down My Production Time?
It's a common fear that strict quality control will create bottlenecks. The truth is the opposite. A well-oiled cGMP system is built for speed and reliability. It’s your best defense against the very mistakes that cause costly production holds in the first place.
Because every step is standardized, documented, and repeatable, there are no surprises. Everything is predictable.
A strong cGMP partner delivers on time precisely because their processes are so dialed in. They aren't getting sidetracked by contamination scares, failed batches, or missing paperwork. That operational discipline is what enables rapid turnarounds and a transparent, reliable timeline, giving your brand a smooth path to market.
Can a Startup Brand Afford a GMP Certified Partner?
Absolutely. In fact, a startup can't afford not to. The best manufacturing partners are structured to support brands at every stage, knowing that today's small pilot batch is tomorrow's massive production run.
You'll want to find a manufacturer that offers the customization and flexibility you need. This means offering a wide range of run sizes, from smaller pilot batches for testing the market to full-scale production runs as you gain traction.
A true partner delivers the same exact level of quality and compliance whether you order a thousand units or a hundred thousand. This empowers you to launch your brand with the same credibility and quality assurance as an industry giant, giving you a serious competitive edge right from day one.
At Triton Nutra Group, we see our cGMP-certified process as the launchpad for your brand's success. We’ve built our entire operation around a partnership mindset, giving you the customization, speed, and reliability you need to bring your vision to life.